Dual effect of phosphatidylinositol (4,5)-bisphosphate PIP(2) on Shaker K(+) [corrected] channels
- PMID: 22932893
- PMCID: PMC3476283
- DOI: 10.1074/jbc.M112.382085
Dual effect of phosphatidylinositol (4,5)-bisphosphate PIP(2) on Shaker K(+) [corrected] channels
Erratum in
- J Biol Chem. 2013 Apr 12;288(15):10951
Abstract
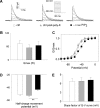
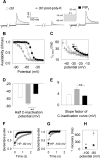
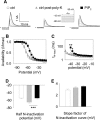
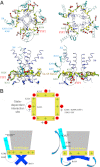
Phosphatidylinositol (4,5)-bisphosphate (PIP(2)) is a phospholipid of the plasma membrane that has been shown to be a key regulator of several ion channels. Functional studies and more recently structural studies of Kir channels have revealed the major impact of PIP(2) on the open state stabilization. A similar effect of PIP(2) on the delayed rectifiers Kv7.1 and Kv11.1, two voltage-gated K(+) channels, has been suggested, but the molecular mechanism remains elusive and nothing is known on PIP(2) effect on other Kv such as those of the Shaker family. By combining giant-patch ionic and gating current recordings in COS-7 cells, and voltage-clamp fluorimetry in Xenopus oocytes, both heterologously expressing the voltage-dependent Shaker channel, we show that PIP(2) exerts 1) a gain-of-function effect on the maximal current amplitude, consistent with a stabilization of the open state and 2) a loss-of-function effect by positive-shifting the activation voltage dependence, most likely through a direct effect on the voltage sensor movement, as illustrated by molecular dynamics simulations.
Figures




References
-
- Hilgemann D. W., Feng S., Nasuhoglu C. (2001) The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE 2001, re19. - PubMed
-
- McLaughlin S., Wang J., Gambhir A., Murray D. (2002) PIP(2) and proteins: interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 31, 151–175 - PubMed
-
- Huang C. L., Feng S., Hilgemann D. W. (1998) Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by G[β][γ]. Nature 391, 803–806 - PubMed
-
- Hilgemann D. W., Ball R. (1996) Regulation of cardiac Na+,Ca2+ exchange, and KATP potassium channels by PIP2. Science 273, 956–959 - PubMed
-
- Zhang H., Craciun L. C., Mirshahi T., Rohács T., Lopes C. M., Jin T., Logothetis D. E. (2003) PIP(2) activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron 37, 963–975 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

