Apolipoprotein E does not cross the blood-cerebrospinal fluid barrier, as revealed by an improved technique for sampling CSF from mice
- PMID: 22933021
- PMCID: PMC3517701
- DOI: 10.1152/ajpregu.00219.2012
Apolipoprotein E does not cross the blood-cerebrospinal fluid barrier, as revealed by an improved technique for sampling CSF from mice
Abstract
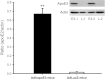
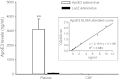
Apolipoprotein E (apoE) is a 34-kDa glycoprotein that is important in lipoprotein metabolism both peripherally and centrally. Because it is primarily produced in the liver, apoE observed in the brain or cerebrospinal fluid (CSF) could have originated in the periphery; i.e., circulating apoE may cross the blood-brain barrier (BBB) and/or enter CSF and be taken up by brain cells. To determine whether this occurs, a second-generation adenovirus encoding human apoE3 was administered intravenously (iv) to C57BL/6J mice, and the detection of human apoE3 in the CSF was used as a surrogate measure of central availability of this protein utilizing an improved method for sampling CSF from mice. This improved technique collects mouse CSF samples with a 92% success rate and consistently yields relatively large volumes of CSF with a very low rate of blood contamination, as determined by molecular assessment of apolipoprotein B, a plasma-derived protein that is absent in the central nervous system. Through this improved method, we demonstrated that in mice receiving the administered apoE3 adenovirus, human apoE3 was expressed at high levels in the liver, leading to high levels of human apoE3 in mouse plasma. In contrast, human apoE3 levels in the CSF, as assessed by a sensitive ELISA, were essentially undetectable in human apoE3 adenovirus-treated mice, and comparable to levels in LacZ adenovirus-treated control mice. These data indicate that apoE in the CSF cannot be derived from the plasma pool and, therefore, must be synthesized locally in the brain.
Figures


References
-
- Beisiegel U. Receptors for triglyceride-rich lipoproteins and their role in lipoprotein metabolism. Curr Opin Lipidol 6: 117–122, 1995 - PubMed
-
- Berzin TM, Zipser BD, Rafii MS, Kuo-Leblanc V, Yancopoulos GD, Glass DJ, Fallon JR, Stopa EG. Agrin and microvascular damage in Alzheimer's disease. Neurobiol Aging 21: 349–355, 2000 - PubMed
-
- Cabana VG, Feng N, Reardon CA, Lukens J, Webb NR, de Beer FC, Getz GS. Influence of apoA-I and apoE on the formation of serum amyloid A-containing lipoproteins in vivo and in vitro. J Lipid Res 45: 317–325, 2004 - PubMed
-
- Carp RI, Davidson AL, Merz PA. A method for obtaining cerebrospinal fluid from mice. Res Vet Sci 12: 499, 1971 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

