Loss of ACE2 exaggerates high-calorie diet-induced insulin resistance by reduction of GLUT4 in mice
- PMID: 22933108
- PMCID: PMC3526031
- DOI: 10.2337/db12-0177
Loss of ACE2 exaggerates high-calorie diet-induced insulin resistance by reduction of GLUT4 in mice
Abstract
ACE type 2 (ACE2) functions as a negative regulator of the renin-angiotensin system by cleaving angiotensin II (AII) into angiotensin 1-7 (A1-7). This study assessed the role of endogenous ACE2 in maintaining insulin sensitivity. Twelve-week-old male ACE2 knockout (ACE2KO) mice had normal insulin sensitivities when fed a standard diet. AII infusion or a high-fat, high-sucrose (HFHS) diet impaired glucose tolerance and insulin sensitivity more severely in ACE2KO mice than in their wild-type (WT) littermates. The strain difference in glucose tolerance was not eliminated by an AII receptor type 1 (AT1) blocker but was eradicated by A1-7 or an AT1 blocker combined with the A1-7 inhibitor (A779). The expression of GLUT4 and a transcriptional factor, myocyte enhancer factor (MEF) 2A, was dramatically reduced in the skeletal muscles of the standard diet-fed ACE2KO mice. The expression of GLUT4 and MEF2A was increased by A1-7 in ACE2KO mice and decreased by A779 in WT mice. A1-7 enhanced upregulation of MEF2A and GLUT4 during differentiation of myoblast cells. In conclusion, ACE2 protects against high-calorie diet-induced insulin resistance in mice. This mechanism may involve the transcriptional regulation of GLUT4 via an A1-7-dependent pathway.
Figures


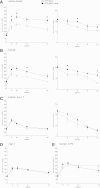

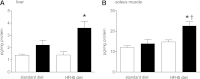

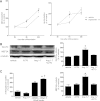
Comment in
-
Response to comment on: Takeda et al. Loss of ACE2 exaggerates high-calorie diet-induced insulin resistance by reduction of GLUT4 in mice. Diabetes 2013;62:223-233.Diabetes. 2013 Jul;62(7):e10. doi: 10.2337/db13-0505. Diabetes. 2013. PMID: 23801721 Free PMC article. No abstract available.
-
Comment on: Takeda et al. Loss of ACE2 exaggerates high-calorie diet-induced insulin resistance by reduction of GLUT4 in mice. Diabetes 2013;62:223-233.Diabetes. 2013 Jul;62(7):e9. doi: 10.2337/db13-0389. Diabetes. 2013. PMID: 23801722 Free PMC article. No abstract available.
References
-
- Abuissa H, Jones PG, Marso SP, O’Keefe JH., Jr Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. J Am Coll Cardiol 2005;46:821–826 - PubMed
-
- Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 2000;275:33238–33243 - PubMed
-
- Yamamoto K, Ohishi M, Katsuya T, et al. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension 2006;47:718–726 - PubMed
-
- Shiota A, Yamamoto K, Ohishi M, et al. Loss of ACE2 accelerates time-dependent glomerular and tubulointerstitial damage in streptozotocin-induced diabetic mice. Hypertens Res 2010;33:298–307 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

