PPARδ activation protects endothelial function in diabetic mice
- PMID: 22933110
- PMCID: PMC3501853
- DOI: 10.2337/db12-0117
PPARδ activation protects endothelial function in diabetic mice
Abstract
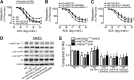
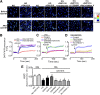
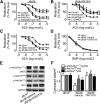
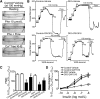
Recent evidence highlights the therapeutic potential of peroxisome proliferator-activated receptor-δ (PPARδ) agonists to increase insulin sensitivity in diabetes. However, the role of PPARδ in regulating vascular function is incompletely characterized. We investigate whether PPARδ activation improves endothelial function in diabetic and obese mice. PPARδ knockout (KO) and wild-type (WT) mice fed with high-fat diet and db/db mice were used as diabetic mouse models, compared with PPARδ KO and WT mice on normal diet and db/m(+) mice. Endothelium-dependent relaxation (EDR) was measured by wire myograph. Flow-mediated vasodilatation (FMD) was measured by pressure myograph. Nitric oxide (NO) production was examined in primary endothelial cells from mouse aortae. PPARδ agonist GW1516 restored EDRs in mouse aortae under high-glucose conditions or in db/db mouse aortae ex vivo. After oral treatment with GW1516, EDRs in aortae and FMDs in mesenteric resistance arteries were improved in obese mice in a PPARδ-specific manner. The effects of GW1516 on endothelial function were mediated through phosphatidylinositol 3-kinase (PI3K) and Akt with a subsequent increase of endothelial nitric oxide synthase (eNOS) activity and NO production. The current study demonstrates an endothelial-protective effect of PPARδ agonists in diabetic mice through PI3K/Akt/eNOS signaling, suggesting the therapeutic potential of PPARδ agonists for diabetic vasculopathy.
Figures


Similar articles
-
Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5' adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor δ pathway.Arterioscler Thromb Vasc Biol. 2014 Apr;34(4):830-6. doi: 10.1161/ATVBAHA.113.301938. Epub 2014 Jan 30. Arterioscler Thromb Vasc Biol. 2014. PMID: 24482374
-
Paeonol protects against endoplasmic reticulum stress-induced endothelial dysfunction via AMPK/PPARδ signaling pathway.Biochem Pharmacol. 2016 Sep 15;116:51-62. doi: 10.1016/j.bcp.2016.07.013. Epub 2016 Jul 20. Biochem Pharmacol. 2016. PMID: 27449753
-
Hydrogen sulfide improves endothelial dysfunction in hypertension by activating peroxisome proliferator-activated receptor delta/endothelial nitric oxide synthase signaling.J Hypertens. 2018 Mar;36(3):651-665. doi: 10.1097/HJH.0000000000001605. J Hypertens. 2018. PMID: 29084084
-
Peroxisome proliferator-activated receptor δ agonist GW1516 attenuates diet-induced aortic inflammation, insulin resistance, and atherosclerosis in low-density lipoprotein receptor knockout mice.Arterioscler Thromb Vasc Biol. 2014 Jan;34(1):52-60. doi: 10.1161/ATVBAHA.113.301830. Epub 2013 Oct 24. Arterioscler Thromb Vasc Biol. 2014. PMID: 24158519
-
Recent Updates on Peroxisome Proliferator-Activated Receptor δ Agonists for the Treatment of Metabolic Syndrome.Med Chem. 2016;12(1):3-21. doi: 10.2174/1573406411666150525105826. Med Chem. 2016. PMID: 26004782 Review.
Cited by
-
The selective PPAR-delta agonist seladelpar reduces ethanol-induced liver disease by restoring gut barrier function and bile acid homeostasis in mice.Transl Res. 2021 Jan;227:1-14. doi: 10.1016/j.trsl.2020.06.006. Epub 2020 Jun 15. Transl Res. 2021. PMID: 32553670 Free PMC article.
-
The Role of Nrf2 Signaling in PPARβ/δ-Mediated Vascular Protection against Hyperglycemia-Induced Oxidative Stress.Oxid Med Cell Longev. 2018 Jun 25;2018:5852706. doi: 10.1155/2018/5852706. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30046379 Free PMC article.
-
GDF11/BMP11 activates both smad1/5/8 and smad2/3 signals but shows no significant effect on proliferation and migration of human umbilical vein endothelial cells.Oncotarget. 2016 Mar 15;7(11):12063-74. doi: 10.18632/oncotarget.7642. Oncotarget. 2016. PMID: 26919250 Free PMC article.
-
PPAR control of metabolism and cardiovascular functions.Nat Rev Cardiol. 2021 Dec;18(12):809-823. doi: 10.1038/s41569-021-00569-6. Epub 2021 Jun 14. Nat Rev Cardiol. 2021. PMID: 34127848 Review.
-
Black tea protects against hypertension-associated endothelial dysfunction through alleviation of endoplasmic reticulum stress.Sci Rep. 2015 May 15;5:10340. doi: 10.1038/srep10340. Sci Rep. 2015. PMID: 25976123 Free PMC article.
References
-
- Wang YX, Lee CH, Tiep S, et al. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell 2003;113:159–170 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

