Altered MAPK signaling in progressive deterioration of endothelial function in diabetic mice
- PMID: 22933112
- PMCID: PMC3501862
- DOI: 10.2337/db12-0559
Altered MAPK signaling in progressive deterioration of endothelial function in diabetic mice
Abstract
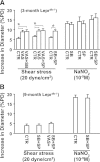
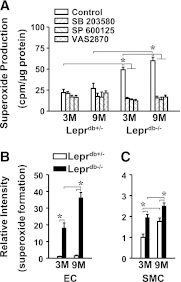
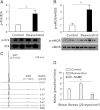
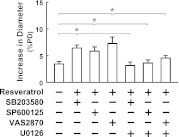
We aimed to investigate specific roles of mitogen-activated protein kinases (MAPK) in the deterioration of endothelial function during the progression of diabetes and the potential therapeutic effects of MAPK inhibitors and agonists in the amelioration of endothelial function. Protein expression and phosphorylation of p38, c-Jun NH(2)-terminal kinase (JNK), and extracellular signal-regulated kinase (Erk) were assessed in mesenteric arteries of 3- (3M) and 9-month-old (9M) male diabetic and control mice. The expression of p38, JNK, and Erk was comparable in all groups of mice, but the phosphorylation of p38 and JNK was increased in 3M and further increased in 9M diabetic mice, whereas the phosphorylation of Erk was substantially reduced in 9M diabetic mice. NADPH oxidase-dependent superoxide production was significantly increased in vessels of two ages of diabetic mice. Inhibition of either p38 with SB203580 or JNK with SP600125 reduced superoxide production and improved shear stress-induced dilation (SSID) in 3M, but not in 9M, diabetic mice. Treating the vessels of 9M diabetic mice with resveratrol increased Erk phosphorylation and shear stress-induced endothelial nitric oxide synthase (eNOS) phosphorylation and activity, but resveratrol alone did not improve SSID. Administration of resveratrol and SB203580 or resveratrol and SP600125 together significantly improved SSID in vessels of 9M diabetic mice. The improved response was prevented by U0126, an Erk inhibitor. Thus, p38/JNK-dependent increase in oxidative stress diminished nitric oxide-mediated dilation in vessels of 3M diabetic mice. Oxidative stress and impaired Erk-dependent activation of eNOS exacerbates endothelial dysfunction in the advanced stage of diabetes.
Figures



References
-
- Hamed S, Brenner B, Abassi Z, Aharon A, Daoud D, Roguin A. Hyperglycemia and oxidized-LDL exert a deleterious effect on endothelial progenitor cell migration in type 2 diabetes mellitus. Thromb Res 2010;126:166–174 - PubMed
-
- Hamed S, Brenner B, Roguin A. Nitric oxide: a key factor behind the dysfunctionality of endothelial progenitor cells in diabetes mellitus type-2. Cardiovasc Res 2011;91:9–15 - PubMed
-
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 2002;23:599–622 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

