Bcl-2 and Bcl-xL suppress glucose signaling in pancreatic β-cells
- PMID: 22933114
- PMCID: PMC3526034
- DOI: 10.2337/db11-1464
Bcl-2 and Bcl-xL suppress glucose signaling in pancreatic β-cells
Abstract
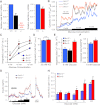
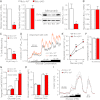
B-cell lymphoma 2 (Bcl-2) family proteins are established regulators of cell survival, but their involvement in the normal function of primary cells has only recently begun to receive attention. In this study, we demonstrate that chemical and genetic loss-of-function of antiapoptotic Bcl-2 and Bcl-x(L) significantly augments glucose-dependent metabolic and Ca(2+) signals in primary pancreatic β-cells. Antagonism of Bcl-2/Bcl-x(L) by two distinct small-molecule compounds rapidly hyperpolarized β-cell mitochondria, increased cytosolic Ca(2+), and stimulated insulin release via the ATP-dependent pathway in β-cell under substimulatory glucose conditions. Experiments with single and double Bax-Bak knockout β-cells established that this occurred independently of these proapoptotic binding partners. Pancreatic β-cells from Bcl-2(-/-) mice responded to glucose with significantly increased NAD(P)H levels and cytosolic Ca(2+) signals, as well as significantly augmented insulin secretion. Inducible deletion of Bcl-x(L) in adult mouse β-cells also increased glucose-stimulated NAD(P)H and Ca(2+) responses and resulted in an improvement of in vivo glucose tolerance in the conditional Bcl-x(L) knockout animals. Our work suggests that prosurvival Bcl proteins normally dampen the β-cell response to glucose and thus reveals these core apoptosis proteins as integrators of cell death and physiology in pancreatic β-cells.
Figures






Comment in
-
Dual Trade of Bcl-2 and Bcl-xL in islet physiology: balancing life and death with metabolism secretion coupling.Diabetes. 2013 Jan;62(1):18-21. doi: 10.2337/db12-1023. Diabetes. 2013. PMID: 23258905 Free PMC article. No abstract available.
Similar articles
-
Bax and Bak jointly control survival and dampen the early unfolded protein response in pancreatic β-cells under glucolipotoxic stress.Sci Rep. 2020 Jul 3;10(1):10986. doi: 10.1038/s41598-020-67755-3. Sci Rep. 2020. PMID: 32620813 Free PMC article.
-
Dual Trade of Bcl-2 and Bcl-xL in islet physiology: balancing life and death with metabolism secretion coupling.Diabetes. 2013 Jan;62(1):18-21. doi: 10.2337/db12-1023. Diabetes. 2013. PMID: 23258905 Free PMC article. No abstract available.
-
Overexpression of Bcl-x(L) in beta-cells prevents cell death but impairs mitochondrial signal for insulin secretion.Am J Physiol Endocrinol Metab. 2000 Feb;278(2):E340-51. doi: 10.1152/ajpendo.2000.278.2.E340. Am J Physiol Endocrinol Metab. 2000. PMID: 10662719
-
Shortcomings of current models of glucose-induced insulin secretion.Diabetes Obes Metab. 2009 Nov;11 Suppl 4:168-79. doi: 10.1111/j.1463-1326.2009.01109.x. Diabetes Obes Metab. 2009. PMID: 19817799 Review.
-
Bcl-2 proteins and calcium signaling: complexity beneath the surface.Oncogene. 2016 Sep 29;35(39):5079-92. doi: 10.1038/onc.2016.31. Epub 2016 Mar 14. Oncogene. 2016. PMID: 26973249 Review.
Cited by
-
Control of insulin secretion by cytochrome C and calcium signaling in islets with impaired metabolism.J Biol Chem. 2014 Jul 4;289(27):19110-9. doi: 10.1074/jbc.M114.556050. Epub 2014 May 19. J Biol Chem. 2014. PMID: 24841202 Free PMC article.
-
A practical guide to genetic engineering of pancreatic β-cells in vivo: getting a grip on RIP and MIP.Islets. 2014;6(3):e944439. doi: 10.4161/19382014.2014.944439. Islets. 2014. PMID: 25322827 Free PMC article. Review.
-
Histone Deacetylase 3 Aggravates Type 1 Diabetes Mellitus by Inhibiting Lymphocyte Apoptosis Through the microRNA-296-5p/Bcl-xl Axis.Front Genet. 2020 Nov 2;11:536854. doi: 10.3389/fgene.2020.536854. eCollection 2020. Front Genet. 2020. PMID: 33240312 Free PMC article.
-
Apoptosis in pancreatic β-islet cells in Type 2 diabetes.Bosn J Basic Med Sci. 2016 Aug 2;16(3):162-79. doi: 10.17305/bjbms.2016.919. Epub 2016 May 22. Bosn J Basic Med Sci. 2016. PMID: 27209071 Free PMC article. Review.
-
Modulation of the Apoptosis Gene Bcl-x Function Through Alternative Splicing.Front Genet. 2019 Sep 6;10:804. doi: 10.3389/fgene.2019.00804. eCollection 2019. Front Genet. 2019. PMID: 31552099 Free PMC article. Review.
References
-
- Petros AM, Olejniczak ET, Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta 2004;1644:83–94 - PubMed
-
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008;9:47–59 - PubMed
-
- Emamaullee JA, Shapiro AM. Interventional strategies to prevent beta-cell apoptosis in islet transplantation. Diabetes 2006;55:1907–1914 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

