Synthetic lethality of PARP and NAMPT inhibition in triple-negative breast cancer cells
- PMID: 22933245
- PMCID: PMC3491838
- DOI: 10.1002/emmm.201201250
Synthetic lethality of PARP and NAMPT inhibition in triple-negative breast cancer cells
Abstract
PARP inhibitors have been proposed as a potential targeted therapy for patients with triple-negative (ER-, PR-, HER2-negative) breast cancers. However, it is as yet unclear as to whether single agent or combination therapy using PARP inhibitors would be most beneficial. To better understand the mechanisms that determine the response to PARP inhibitors, we investigated whether enzymes involved in metabolism of the PARP substrate, β-NAD(+) , might alter the response to a clinical PARP inhibitor. Using an olaparib sensitization screen in a triple-negative (TN) breast cancer model, we identified nicotinamide phosphoribosyltransferase (NAMPT) as a non-redundant modifier of olaparib response. NAMPT is a rate-limiting enzyme involved in the generation of the PARP substrate β-NAD(+) and the suppression of β-NAD(+) levels by NAMPT inhibition most likely explains these observations. Importantly, the combination of a NAMPT small molecule inhibitor, FK866, with olaparib inhibited TN breast tumour growth in vivo to a greater extent than either single agent alone suggesting that assessing NAMPT/PARP inhibitor combinations for the treatment of TN breast cancer may be warranted.
Copyrights © 2012 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures

PARP inhibitor sensitivity in a panel of TN models. Two-week olaparib survival curves from six-well plate clonogenic assays are shown.
Rank order of drug effect (DE) Z scores from the PARP inhibitor sensitivity screen. Black bars represent Z scores from sample siRNA SMARTPools targeting 44 protein PARPs and β-NAD+-metabolizing genes. The red bar represents the siNAMPT effect, similar in scale to that observed with silencing of BRCA1 (Turner et al, 2008).

A. CAL51 cells were transfected with siRNA in a 96-well plate format and then exposed to olaparib (1 µM final concentration) for 5 days after which cell viability was estimated. Log2 SFs in 1 µM olaparib (normalized to vehicle-treated cells) are shown. Error bars represent the standard error of the mean (SEM) from three independent experiments; *p-values versus siCONTROL1 transfected cells p < 0.0001 (Student's t-test).
B. Validation of siRNA silencing effects. CAL51 cells were transfected with siRNA as shown and cell lysates generated 48 h later. Lysates were Western blotted and immunoprobed using an anti-NAMPT (Cell Signalling) antibody as shown.
C. Validation of siRNA silencing effects using RT-PCR. CAL51 cells were transfected with siRNA as shown and cDNA generated 48 h later. Relative NAMPT mRNA expression (compared to siCONTROL1-transfected cells) is shown.
D,E. Dose–response sensitization to a PARP inhibitor caused by NAMPT silencing. CAL51 (D) or HeLa (E) cells were transfected as before and plated in media containing olaparib as shown. Cell viability was determined after 5 days olaparib exposure using Cell Titre Glo. Survival curves are shown. Error bars represent the SEM from three independent experiments. The effects of siRNA targeting BRCA2 are shown as a positive control; p-values for the siNAMPT-transfected cells versus siCONTROL1-transfected cells p < 0.05 (ANOVA) in CAL51 (D) and Hela (E). See also Supporting Information Table S3.
F,G. Inhibition of NAMPT using a potent inhibitor FK866 sensitizes tumour cells to olaparib. CAL51 (F) or HeLa (G) cells were plated in 96-well plates and exposed to FK866 and/or olaparib, as shown, for 5 days. Cell viability after this time was estimated using Cell Titre Glo (Promega). Survival curves are shown. Error bars represent the SEM from three independent experiments; p-values for CAL51 cells exposed to 10−10–10−7 M FK866 inhibitor and olaparib versus olaparib alone p < 0.05 (ANOVA), all other comparisons returned non significant p-values; p-values for HeLa cells exposed to 10−13–10−7 M FK866 inhibitor and olaparib versus olaparib alone p < 0.05 (ANOVA). See also Supporting Information Table S3.
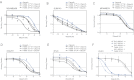
A-E. Sensitivity of the cell lines MDAMB468 (A), SUM149 (B), MDAMB231 (C), HS578T (D) and BT20 (E) was assessed as in Fig 3.
F. Inhibition of NAMPT using the potent inhibitor FK866 sensitizes DLD1 BRCA2−/− tumour cells to olaparib. Viability was measured after 14 days of continuous exposure to FK866/olaparib. Error bars represent the SEM from three independent experiments. ANOVA analysis tables are shown in Supporting Information Table S4.

Cellular β-NAD+ levels in cells exposed to olaparib and/or FK866. CAL51 cells were exposed to FK866 and/or olaparib for 48 h after which β-NAD+ levels were estimated using an NAD/NADH Assay Kit (Abcam).
Nicotinic acid (NA) rescues the combined effect of olaparib and FK866. CAL51 cells were exposed to olaparib and FK866 as shown in addition to 10 µM NA and cell viability estimated after 6 days drug exposure. Error bars represent the SEM from three independent experiments; p-value for CAL51 cells treated with FK866/olaparib versus FK866 inhibitor/olaparib plus 10 µM NA, p < 0.05 (ANOVA).
FK866 exacerbates levels of γH2AX caused by olaparib. CAL51 cells were exposed to FK866 and/or olaparib for 48 h and cell lysates generated and immunoblotted for total and γH2AX.
Quantification of γH2AX foci in CAL51 cells. Cells were exposed to FK866 and/or olaparib as in (C). Bar chart shows the median number of cells with >5 γH2AX foci per nucleus. Error bars represent three standard deviations of the mean; *p-values for CAL51 cells treated with combination of FK866/olaparib versus olaparib alone p < 0.05 (Student's t-test); ns, not significant p > 0.05.
Combination of FK866 inhibitor and olaparib causes an increase in apoptosis as measured by caspase 3/7 activity. Cells were treated as in (C) and caspase 3/7 activity assessed as in the Materials and Methods section. p-values for CAL51 cells treated with 0.01 µM FK866 and olaparib versus olaparib alone p < 0.05 (ANOVA), all other comparisons returned non-significant p-values.
Frequency of apoptotic cells in CAL51 cells exposed to FK866 and/or olaparib. Cells were treated as in (C) and the frequency of Annexin V-positive cells estimated by FACS analysis.
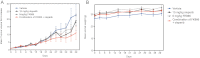
The therapeutic effect of each therapy compared to vehicle-treated mice is shown for olaparib alone, FK866 alone and the olaparib/FK866 combination. Each data point represents the mean increase in tumour volume after the instigation of treatment and error bars represent SEM, where n for each cohort = 10 animals; *p < 0.05 (repeated measures ANOVA with post-test) comparing the FK866/olaparib combination arm versus each other cohort, refer to Supporting Information Table S5 for statistical analysis.
Body weight in each cohort.
References
-
- Daniel RA, Rozanska AL, Mulligan EA, Drew Y, Thomas HD, Castelbuono DJ, Hostomsky Z, Plummer ER, Tweddle DA, Boddy AV, et al. Central nervous system penetration and enhancement of temozolomide activity in childhood medulloblastoma models by poly(ADP-ribose) polymerase inhibitor AG-014699. Br J Cancer. 2010;103:1588–1596. - PMC - PubMed
-
- Drevs J, Loser R, Rattel B, Esser N. Antiangiogenic potency of FK866/K22.175, a new inhibitor of intracellular NAD biosynthesis, in murine renal cell carcinoma. Anticancer Res. 2003;23:4853–4858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

