A novel assay for antibody-dependent cell-mediated cytotoxicity against HIV-1- or SIV-infected cells reveals incomplete overlap with antibodies measured by neutralization and binding assays
- PMID: 22933282
- PMCID: PMC3486484
- DOI: 10.1128/JVI.01650-12
A novel assay for antibody-dependent cell-mediated cytotoxicity against HIV-1- or SIV-infected cells reveals incomplete overlap with antibodies measured by neutralization and binding assays
Abstract
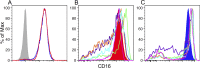
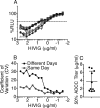
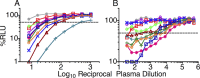
The resistance of human immunodeficiency virus type 1 (HIV-1) to antibody-mediated immunity often prevents the detection of antibodies that neutralize primary isolates of HIV-1. However, conventional assays for antibody functions other than neutralization are suboptimal. Current methods for measuring the killing of virus-infected cells by antibody-dependent cell-mediated cytotoxicity (ADCC) are limited by the number of natural killer (NK) cells obtainable from individual donors, donor-to-donor variation, and the use of nonphysiological targets. We therefore developed an ADCC assay based on NK cell lines that express human or macaque CD16 and a CD4(+) T-cell line that expresses luciferase from a Tat-inducible promoter upon HIV-1 or simian immunodeficiency virus (SIV) infection. NK cells and virus-infected targets are mixed in the presence of serial plasma dilutions, and ADCC is measured as the dose-dependent loss of luciferase activity. Using this approach, ADCC titers were measured in plasma samples from HIV-infected human donors and SIV-infected macaques. For the same plasma samples paired with the same test viruses, this assay was approximately 2 orders of magnitude more sensitive than optimized assays for neutralizing antibodies-frequently allowing the measurement of ADCC in the absence of detectable neutralization. Although ADCC correlated with other measures of Env-specific antibodies, neutralizing and gp120 binding titers did not consistently predict ADCC activity. Hence, this assay affords a sensitive method for measuring antibodies capable of directing ADCC against HIV- or SIV-infected cells expressing native conformations of the viral envelope glycoprotein and reveals incomplete overlap of the antibodies that direct ADCC and those measured in neutralization and binding assays.
Figures



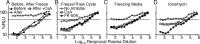

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

