Oral gene application using chitosan-DNA nanoparticles induces transferable tolerance
- PMID: 22933401
- PMCID: PMC3491548
- DOI: 10.1128/CVI.00186-12
Oral gene application using chitosan-DNA nanoparticles induces transferable tolerance
Abstract
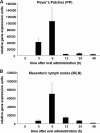
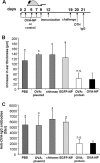
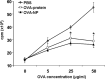
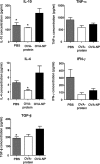
Oral tolerance is a promising approach to induce unresponsiveness to various antigens. The development of tolerogenic vaccines could be exploited in modulating the immune response in autoimmune disease and allograft rejection. In this study, we investigated a nonviral gene transfer strategy for inducing oral tolerance via antigen-encoding chitosan-DNA nanoparticles (NP). Oral application of ovalbumin (OVA)-encoding chitosan-DNA NP (OVA-NP) suppressed the OVA-specific delayed-type hypersensitivity (DTH) response and anti-OVA antibody formation, as well as spleen cell proliferation following OVA stimulation. Cytokine expression patterns following OVA stimulation in vitro showed a shift from a Th1 toward a Th2/Th3 response. The OVA-NP-induced tolerance was transferable from donor to naïve recipient mice via adoptive spleen cell transfer and was mediated by CD4(+)CD25(+) T cells. These findings indicate that nonviral oral gene transfer can induce regulatory T cells for antigen-specific immune modulation.
Figures

Similar articles
-
Induction of ovalbumin-specific tolerance by oral administration of Lactococcus lactis secreting ovalbumin.Gastroenterology. 2007 Aug;133(2):517-28. doi: 10.1053/j.gastro.2007.04.073. Epub 2007 May 3. Gastroenterology. 2007. PMID: 17681173
-
Oral tolerance induction with antigen conjugated to cholera toxin B subunit generates both Foxp3+CD25+ and Foxp3-CD25- CD4+ regulatory T cells.J Immunol. 2006 Dec 1;177(11):7634-44. doi: 10.4049/jimmunol.177.11.7634. J Immunol. 2006. PMID: 17114433
-
Targeting the allergen to oral dendritic cells with mucoadhesive chitosan particles enhances tolerance induction.Allergy. 2009 Jul;64(7):1003-13. doi: 10.1111/j.1398-9995.2009.01945.x. Epub 2009 Feb 12. Allergy. 2009. PMID: 19220212
-
CD4+CD25-mTGFbeta+ T cells induced by nasal application of ovalbumin transfer tolerance in a therapeutic model of asthma.Int Immunol. 2011 Jan;23(1):17-27. doi: 10.1093/intimm/dxq453. Epub 2010 Dec 1. Int Immunol. 2011. PMID: 21123830
-
Mechanism of oral tolerance induction to therapeutic proteins.Adv Drug Deliv Rev. 2013 Jun 15;65(6):759-73. doi: 10.1016/j.addr.2012.10.013. Epub 2012 Nov 2. Adv Drug Deliv Rev. 2013. PMID: 23123293 Free PMC article. Review.
Cited by
-
Immune Modulation with Oral DNA/RNA Nanoparticles.Pharmaceutics. 2025 May 4;17(5):609. doi: 10.3390/pharmaceutics17050609. Pharmaceutics. 2025. PMID: 40430900 Free PMC article. Review.
-
Mucosal immunization with high-mobility group box 1 in chitosan enhances DNA vaccine-induced protection against coxsackievirus B3-induced myocarditis.Clin Vaccine Immunol. 2013 Nov;20(11):1743-51. doi: 10.1128/CVI.00466-13. Epub 2013 Sep 11. Clin Vaccine Immunol. 2013. PMID: 24027262 Free PMC article.
-
Nanoparticles for the Induction of Antigen-Specific Immunological Tolerance.Front Immunol. 2018 Feb 20;9:230. doi: 10.3389/fimmu.2018.00230. eCollection 2018. Front Immunol. 2018. PMID: 29515571 Free PMC article. Review.
-
Chitosan-zein nano-in-microparticles capable of mediating in vivo transgene expression following oral delivery.J Control Release. 2017 Mar 10;249:150-161. doi: 10.1016/j.jconrel.2017.01.035. Epub 2017 Jan 31. J Control Release. 2017. PMID: 28153762 Free PMC article.
-
Nano-based approaches for the treatment of neuro-immunological disorders: a special emphasis on multiple sclerosis.Discov Nano. 2024 Oct 28;19(1):171. doi: 10.1186/s11671-024-04135-0. Discov Nano. 2024. PMID: 39466516 Free PMC article. Review.
References
-
- Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. 2004. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control Release 100:5–28 - PubMed
-
- Bellmann K, et al. 1997. Intervention in autoimmune diabetes by targeting the gut immune system. Int. J. Immunopharmacol. 19:573–577 - PubMed
-
- Bivas-Benita M, et al. 2004. Pulmonary delivery of chitosan-DNA nanoparticles enhances the immunogenicity of a DNA vaccine encoding HLA-A*0201-restricted T-cell epitopes of Mycobacterium tuberculosis. Vaccine 22:1609–1615 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

