Transgenic analysis of signaling pathways required for Xenopus tadpole spinal cord and muscle regeneration
- PMID: 22933404
- PMCID: PMC3442130
- DOI: 10.1002/ar.22437
Transgenic analysis of signaling pathways required for Xenopus tadpole spinal cord and muscle regeneration
Abstract
The Xenopus tadpole has the capacity fully to regenerate its tail after amputation. Previously, we have established that this regeneration process requires the operation of several signaling pathways including the bone morphogenic protein, Wnt, and Fgf pathways. Here, we have addressed the signaling requirements for spinal cord and muscle regeneration in a tissue-specific manner. Two methods were used namely grafts of transgenic spinal cord to a wild type host, and the use of the Tet-on conditional transgenic system to express inhibitors in the individual tissues. For the grafting experiments, the tail was amputated through the graft, which contained a temperature inducible inhibitor of the Wnt-β-catenin pathway. For the Tet-on experiments, treatment with doxycycline was used to induce cell autonomous inhibitors of the Wnt-β-catenin or the Fgf pathway in either spinal cord or muscle. The results show that both spinal cord and muscle regeneration depend on both the Wnt-β-catenin and the Fgf pathways. This experimental design also enables us to observe the effect of inhibition of regeneration of one tissue on the regeneration of the others. Regardless of the method of inhibition, we find that reduction of spinal cord regeneration reduces regeneration of other parts of the tail, including the myotomal muscles. In contrast, reduction of muscle regeneration has no effect on the regeneration of the spinal cord. In common with other regeneration systems, this indicates that soluble factors from the spinal cord are needed to promote the regeneration of the other tissues in the tail.
Copyright © 2012 Wiley Periodicals, Inc.
Figures
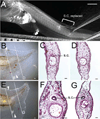


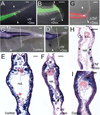
Similar articles
-
Requirement for Wnt and FGF signaling in Xenopus tadpole tail regeneration.Dev Biol. 2008 Apr 15;316(2):323-35. doi: 10.1016/j.ydbio.2008.01.032. Epub 2008 Feb 7. Dev Biol. 2008. PMID: 18329638
-
Notochord-derived hedgehog is essential for tail regeneration in Xenopus tadpole.BMC Dev Biol. 2014 Jun 18;14:27. doi: 10.1186/1471-213X-14-27. BMC Dev Biol. 2014. PMID: 24941877 Free PMC article.
-
Different requirement for Wnt/β-catenin signaling in limb regeneration of larval and adult Xenopus.PLoS One. 2011;6(7):e21721. doi: 10.1371/journal.pone.0021721. Epub 2011 Jul 26. PLoS One. 2011. PMID: 21814549 Free PMC article.
-
Cellular and molecular mechanisms of regeneration in Xenopus.Philos Trans R Soc Lond B Biol Sci. 2004 May 29;359(1445):745-51. doi: 10.1098/rstb.2004.1463. Philos Trans R Soc Lond B Biol Sci. 2004. PMID: 15293801 Free PMC article. Review.
-
Tail regeneration in the Xenopus tadpole.Dev Growth Differ. 2007 Feb;49(2):155-61. doi: 10.1111/j.1440-169X.2007.00912.x. Dev Growth Differ. 2007. PMID: 17335436 Review.
Cited by
-
Tail Tales: What We Have Learned About Regeneration from Xenopus Laevis Tadpoles.Int J Mol Sci. 2024 Oct 29;25(21):11597. doi: 10.3390/ijms252111597. Int J Mol Sci. 2024. PMID: 39519148 Free PMC article. Review.
-
Unique advantages of zebrafish larvae as a model for spinal cord regeneration.Front Mol Neurosci. 2022 Sep 7;15:983336. doi: 10.3389/fnmol.2022.983336. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36157068 Free PMC article. Review.
-
Selective depolarization of transmembrane potential alters muscle patterning and muscle cell localization in Xenopus laevis embryos.Int J Dev Biol. 2015;59(7-9):303-11. doi: 10.1387/ijdb.150198ml. Int J Dev Biol. 2015. PMID: 26198143 Free PMC article.
-
Studying mechanisms of regeneration in amphibian and reptilian vertebrate models.Anat Rec (Hoboken). 2012 Oct;295(10):1529-31. doi: 10.1002/ar.22541. Epub 2012 Aug 29. Anat Rec (Hoboken). 2012. PMID: 22933304 Free PMC article. Review. No abstract available.
-
Bacterial lipopolysaccharides can initiate regeneration of the Xenopus tadpole tail.iScience. 2021 Oct 14;24(11):103281. doi: 10.1016/j.isci.2021.103281. eCollection 2021 Nov 19. iScience. 2021. PMID: 34765912 Free PMC article.
References
-
- Adams DS, Masi A, Levin M. H+ pump dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007;134:1323–1335. - PubMed
-
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. - PubMed
-
- Beck CW, Christen B, Barker D, Slack JMW. Temporal requirement for bone morphogenetic proteins in regeneration of the tail and limb of Xenopus tadpoles. Mech Dev. 2006;123:674–688. - PubMed
-
- Beck CW, Christen B, Slack JMW. Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Dev Cell. 2003a;5:429–439. - PubMed
-
- Beck CW, Christen B, Slack JMW. Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Dev Cell. 2003b;5:429–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

