Serum 25-hydroxyvitamin D response to vitamin D3 supplementation 50,000 IU monthly in youth with HIV-1 infection
- PMID: 22933542
- PMCID: PMC3485594
- DOI: 10.1210/jc.2012-2600
Serum 25-hydroxyvitamin D response to vitamin D3 supplementation 50,000 IU monthly in youth with HIV-1 infection
Abstract
Context: Vitamin D deficiency and insufficiency occur frequently in youth with HIV infection, particularly among those receiving the antiretroviral drug efavirenz. Optimal vitamin D dosing for treatment is unclear.
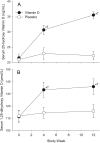
Objective: Our objective was to evaluate safety and measure change in 25-hydroxyvitamin D (25-OHD) concentration from baseline to study wk 4 and 12 during treatment with vitamin D(3), 50,000 IU monthly.
Design, setting, and participants: We conducted a randomized double-blind, placebo-controlled multicenter trial of HIV-infected youth ages 18-24 yr, with viral load below 5000 copies/ml, on stable antiretroviral therapy.
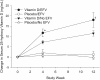
Intervention: INTERVENTION included vitamin D(3), 50,000 IU (n = 102), or matching placebo (n = 101) administered in three directly observed oral doses at monthly intervals.
Results: At baseline, mean (sd) age was 20.9 (2.0) yr; 37% were female and 52% African-American, and 54% were vitamin D deficient/insufficient (25-OHD < 20 ng/ml), with no randomized group differences. Of evaluable participants vitamin D deficient/insufficient at baseline who were administered vitamin D, 43 of 46 (93%) had sufficient 25-OHD by wk 12. Vitamin D supplementation increased 25-OHD serum concentration from a baseline of 21.9 (13.3) to 35.9 (19.1) ng/ml at wk 12 (P < 0.001) with no change for placebo. Although use of the antiretroviral efavirenz was associated with lower baseline 25-OHD concentration, efavirenz did not diminish the response to vitamin D supplementation. There was no treatment-related toxicity.
Conclusions: Supplementation with vitamin D(3) 50,000 IU monthly for three doses was safe. Increases in 25-OHD occurred in treated participants regardless of antiretroviral regimen.
Figures
References
-
- Stephensen CB, Marquis GS, Kruzich LA, Douglas SD, Aldrovandi GM, Wilson CM. 2006. Vitamin D status in adolescents and young adults with HIV infection. Am J Clin Nutr 83:1135–1141 - PubMed
-
- Arpadi SM, McMahon D, Abrams EJ, Bamji M, Purswani M, Engelson ES, Horlick M, Shane E. 2009. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics [Erratum (2009) 123:1437] 123:e121–e126 - PMC - PubMed
-
- Adams JS, Kantorovich V, Wu C, Javanbakht M, Hollis BW. 1999. Resolution of vitamin D insufficiency in osteopenic patients results in rapid recovery of bone mineral density. J Clin Endocrinol Metab 84:2729–2730 - PubMed
-
- Aloia JF, Patel M, Dimaano R, Li-Ng M, Talwar SA, Mikhail M, Pollack S, Yeh JK, Aloia JF, Patel M, Dimaano R, Li-Ng M, Talwar SA, Mikhail M, Pollack S, Yeh JK. 2008. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr 87:1952–1958 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1-RR02517/RR/NCRR NIH HHS/United States
- U01 HD040533/HD/NICHD NIH HHS/United States
- M01-RR00188/RR/NCRR NIH HHS/United States
- U01 HD040497/HD/NICHD NIH HHS/United States
- M01 RR000188/RR/NCRR NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- M01 RR010710/RR/NCRR NIH HHS/United States
- M01RR020359/RR/NCRR NIH HHS/United States
- U01 HD 040533/HD/NICHD NIH HHS/United States
- UL1-RR025014/RR/NCRR NIH HHS/United States
- U01 HD040474/HD/NICHD NIH HHS/United States
- M01-RR10710/RR/NCRR NIH HHS/United States
- UL1 RR025014/RR/NCRR NIH HHS/United States
- U01 A1068632/PHS HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- M01 RR020359/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

