IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration
- PMID: 22933625
- PMCID: PMC3448810
- DOI: 10.4049/jimmunol.1103180
IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration
Abstract
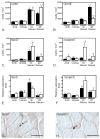
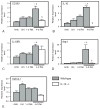
We examined the function of IL-10 in regulating changes in macrophage phenotype during muscle growth and regeneration following injury. Our findings showed that the Th1 cytokine response in inflamed muscle is characterized by high levels of expression of CD68, CCL-2, TNF-α, and IL-6 at 1 d postinjury. During transition to the Th2 cytokine response, expression of those transcripts declined, whereas CD163, IL-10, IL-10R1, and arginase-1 increased. Ablation of IL-10 amplified the Th1 response at 1 d postinjury, causing increases in IL-6 and CCL2, while preventing a subsequent increase in CD163 and arginase-1. Reductions in muscle fiber damage that normally occurred between 1 and 4 d postinjury did not occur in IL-10 mutants. In addition, muscle regeneration and growth were greatly slowed by loss of IL-10. Furthermore, myogenin expression increased in IL-10 mutant muscle at 1 d postinjury, suggesting that the mutation amplified the transition from the proliferative to the early differentiation stages of myogenesis. In vitro assays showed that stimulation of muscle cells with IL-10 had no effect on cell proliferation or expression of MyoD or myogenin. However, coculturing muscle cells with macrophages activated with IL-10 to the M2 phenotype increased myoblast proliferation without affecting MyoD or myogenin expression, showing that M2 macrophages promote the early, proliferative stage of myogenesis. Collectively, these data show that IL-10 plays a central role in regulating the switch of muscle macrophages from a M1 to M2 phenotype in injured muscle in vivo, and this transition is necessary for normal growth and regeneration of muscle.
Figures





References
-
- Smith JK, Grisham MB, Granger DN, Korthuis RJ. Free radical defense mechanisms and neutrophil infiltration in postischemic skeletal muscle. Am J Physiol. 1989;256:H789–793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

