A role of fluoride on free radical generation and oxidative stress in BV-2 microglia cells
- PMID: 22933830
- PMCID: PMC3425889
- DOI: 10.1155/2012/102954
A role of fluoride on free radical generation and oxidative stress in BV-2 microglia cells
Abstract
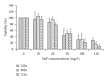
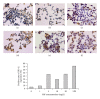
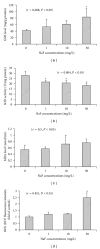
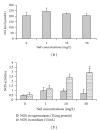
The generation of ROS and lipid peroxidation has been considered to play an important role in the pathogenesis of chronic fluoride toxicity. In the present study, we observed that fluoride activated BV-2 microglia cell line by observing OX-42 expression in immunocytochemistry. Intracellular superoxide dismutase (SOD), glutathione (GSH), malondialdehyde (MDA), reactive oxygen species (ROS), superoxide anions (O(2)(∙-)), nitric oxide synthase (NOS), nitrotyrosine (NT) and nitric oxide (NO), NOS in cell medium were determined for oxidative stress assessment. Our study found that NaF of concentration from 5 to 20 mg/L can stimuli BV-2 cells to change into activated microglia displaying upregulated OX-42 expression. SOD activities significantly decreased in fluoride-treated BV-2 cells as compared with control, and MDA concentrations and contents of ROS and O(2)(∙-) increased in NaF-treated cells. Activities of NOS in cells and medium significantly increased with fluoride concentrations in a dose-dependent manner. NT concentrations also increased significantly in 10 and 50 mg/L NaF-treated cells compared with the control cells. Our present study demonstrated that toxic effects of fluoride on the central nervous system possibly partly ascribed to activiting of microglia, which enhanced oxidative stress induced by ROS and reactive nitrogen species.
Figures

References
-
- Chachra D, Vieira APGF, Grynpas MD. Fluoride and mineralized tissues. Critical Reviews in Biomedical Engineering. 2008;36(2-3):183–223. - PubMed
-
- Ozsvath DL. Fluoride and environmental health: a review. Reviews in Environmental Science and Biotechnology. 2009;8(1):59–79.
-
- Lu Y, Sun ZR, Wu LN, Wang X, Lu W, Liu SS. Effect of high-fluoride water on intelligence in children. Fluoride. 2000;33(2):74–78.
-
- Zhao LB, Liang GH, Zhang DN, Wu XR. Effect of a high fluoride water supply on children’s intelligence. Fluoride. 1996;29(4):190–192.
-
- Li XS, Zhi JL, Gao RO. Effect of fluoride exposure on intelligence in children. Fluoride. 1995;28(4):189–192.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

