Innate immune cells in liver inflammation
- PMID: 22933833
- PMCID: PMC3425885
- DOI: 10.1155/2012/949157
Innate immune cells in liver inflammation
Abstract
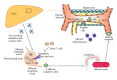
Innate immune system is the first line of defence against invading pathogens that is critical for the overall survival of the host. Human liver is characterised by a dual blood supply, with 80% of blood entering through the portal vein carrying nutrients and bacterial endotoxin from the gastrointestinal tract. The liver is thus constantly exposed to antigenic loads. Therefore, pathogenic microorganism must be efficiently eliminated whilst harmless antigens derived from the gastrointestinal tract need to be tolerized in the liver. In order to achieve this, the liver innate immune system is equipped with multiple cellular components; monocytes, macrophages, granulocytes, natural killer cells, and dendritic cells which coordinate to exert tolerogenic environment at the same time detect, respond, and eliminate invading pathogens, infected or transformed self to mount immunity. This paper will discuss the innate immune cells that take part in human liver inflammation, and their roles in both resolution of inflammation and tissue repair.
Figures



References
-
- O’Farrelly C, Doherty DG. Liver Immunology Principles and Practice. chapter 1. 2007. A short primer on fundamental immunology; pp. 15–24.
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. - PubMed
-
- Lalor PF, Adams DH. The liver: a model of organ-specific lymphocyte recruitment. Expert Reviews in Molecular Medicine. 2002;4(2):1–16. - PubMed
-
- Gao B, Jeong WI, Tian Z. Liver: an organ with predominant innate immunity. Hepatology. 2008;47(2):729–736. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

