Murine corneal stroma cells inhibit LPS-induced dendritic cell maturation partially through TGF-β2 secretion in vitro
- PMID: 22933838
- PMCID: PMC3429355
Murine corneal stroma cells inhibit LPS-induced dendritic cell maturation partially through TGF-β2 secretion in vitro
Abstract
Purpose: The peripheral cornea contains mature and immature resident dendritic cells (DCs) while the central cornea is exclusively equipped with immature DCs. There must be some factors that cause immature DCs. This study investigated whether corneal stroma cells (CSCs) inhibit DC maturation by secreting cytokines.
Methods: The messenger ribonucleic acid (mRNA) and protein level of transforming growth factor beta 2 (TGF-β(2)) was analyzed using reverse transcription polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA). Immature DCs were induced to mature in the presence of lipopolysaccharide (LPS) and with concentrations of CSC culture supernatant (containing and not containing neutralizing TGF-β(2) antibodies). Then, the DC phenotypic and functional maturation were analyzed.
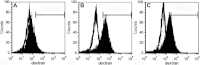
Results: CSCs exhibited positive expressions of TGF-β(2) mRNA and secreted high concentrations of TGF-β(2) protein. In the presence of LPS, DCs, which were treated with a CSC culture supernatant, displayed reduced expressions of cluster of differentiation 80 (CD80), CD86, and major histocompatibility complex II (MHC II) in a dose-dependent manner. Moreover, treated DCs showed lower T-cell stimulation capacity and a higher endocytosis function. However, these phenotypic and functional modifications were partially reversed after the application of neutralizing TGF-β(2) antibodies.
Conclusions: This study demonstrates that CSCs can partially inhibit LPS-induced DC maturation through TGF-β(2) secretion in vitro.
Figures





Similar articles
-
Murine corneal stroma cells suppress bone marrow-derived dendritic cells maturation in vitro.Chin Med J (Engl). 2012 Jun;125(11):2041-7. Chin Med J (Engl). 2012. PMID: 22884074
-
Suppressive effect of aqueous humor on lipopolysaccharide-induced dendritic cell maturation.Jpn J Ophthalmol. 2011 Sep;55(5):558-564. doi: 10.1007/s10384-011-0060-0. Epub 2011 Jul 20. Jpn J Ophthalmol. 2011. PMID: 21773750
-
Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation.Blood. 2001 Sep 1;98(5):1512-23. doi: 10.1182/blood.v98.5.1512. Blood. 2001. PMID: 11520802
-
The role of dendritic cells in the mechanism of action of a peptide that ameliorates lupus in murine models.Immunology. 2009 Sep;128(1 Suppl):e395-405. doi: 10.1111/j.1365-2567.2008.02988.x. Epub 2008 Nov 24. Immunology. 2009. PMID: 19040426 Free PMC article.
-
Potential of targeting TGF-β for organ transplant patients.Future Med Chem. 2013 Mar;5(3):281-9. doi: 10.4155/fmc.12.215. Future Med Chem. 2013. PMID: 23464518 Free PMC article. Review.
Cited by
-
TGFBR3 dependent mechanism of TGFB2 in smooth muscle cell differentiation and implications for TGFB2-related aortic aneurysm.Stem Cells Transl Med. 2025 Mar 18;14(3):szae101. doi: 10.1093/stcltm/szae101. Stem Cells Transl Med. 2025. PMID: 40139558 Free PMC article.
-
Roles of biomaterials in modulating the innate immune response in ocular therapy.Front Drug Deliv. 2023 Feb 15;3:1077253. doi: 10.3389/fddev.2023.1077253. eCollection 2023. Front Drug Deliv. 2023. PMID: 40838059 Free PMC article. Review.
-
Contribution of corneal neovascularization to dendritic cell migration into the central area during human corneal infection.PLoS One. 2014 Oct 9;9(10):e109859. doi: 10.1371/journal.pone.0109859. eCollection 2014. PLoS One. 2014. PMID: 25299318 Free PMC article.
References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. - PubMed
-
- Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. - PubMed
-
- Steinman RM. The control of immunity and tolerance by dendritic cell. Pathol Biol (Paris) 2003;51:59–60. - PubMed
-
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. - PubMed
-
- Niederkorn JY. The immune privilege of corneal allografts. Transplantation. 1999;67:1503–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
