Fluorescence imaging agents in cancerology
- PMID: 22933906
- PMCID: PMC3423689
- DOI: 10.2478/v10019-010-0031-y
Fluorescence imaging agents in cancerology
Abstract
Background: One of the major challenges in cancer therapy is to improve early detection and prevention using novel targeted cancer diagnostics. Detection requests specific recognition. Tumor markers have to be ideally present on the surface of cancer cells. Their targeting with ligands coupled to imaging agents make them visible/detectable.
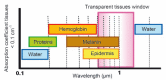
Conclusions: Fluorescence imaging is a newly emerging technology which is becoming a complementary medical method for cancer diagnosis. It allows detection with a high spatio-temporal resolution of tumor markers in small animals and in clinical studies. In this review, we focus on the recent outcome of basic studies in the design of new approaches (probes and devices) used to detect tumor cells by fluorescence imaging.
Keywords: Photonic imaging; apramers; cancerology; fluorescence; smart probes.
Figures

References
-
- Sodee DB, Conant R, Chalfant M, Miron S, Klein E, Bahnson R, et al. Preliminary imaging results using In-111 labeled CYT-356 (Prostascint) in the detection of recurrent prostate cancer. Clin Nucl Med. 1996;21:759–67. - PubMed
-
- Nanus DM, Milowsky MI, Kostakoglu L, Smith-Jones PM, Vallabahajosula S, Goldsmith SJ, et al. Clinical use of monoclonal antibody HuJ591 therapy: targeting prostate specific membrane antigen. J Urol. 2003;170(6 Pt 2):S84–8. discussion S88–9. - PubMed
-
- Abdel-Nabi H, Doerr RJ, Chan HW, Balu D, Schmelter RF, Maguire RT. In-111-labeled monoclonal antibody immunoscintigraphy in colorectal carcinoma: safety, sensitivity, and preliminary clinical results. Radiology. 1990;175:163–71. - PubMed
-
- Moffat FL, Jr, Pinsky CM, Hammershaimb L, Petrelli NJ, Patt YZ, Whaley FS, et al. Clinical utility of external immunoscintigraphy with the IMMU-4 technetium-99m Fab’ antibody fragment in patients undergoing surgery for carcinoma of the colon and rectum: results of a pivotal, phase III trial. The Immunomedics Study Group. J Clin Oncol. 1996;14:2295–305. - PubMed
-
- Breitz HB, Tyler A, Bjorn MJ, Lesley T, Weiden PL. Clinical experience with Tc-99m nofetumomab merpentan (Verluma) radioimmunoscintigraphy. Clin Nucl Med. 1997;22:615–20. - PubMed
LinkOut - more resources
Full Text Sources

