Cathepsin H indirectly regulates morphogenetic protein-4 (BMP-4) in various human cell lines
- PMID: 22933963
- PMCID: PMC3423750
- DOI: 10.2478/v10019-011-0034-3
Cathepsin H indirectly regulates morphogenetic protein-4 (BMP-4) in various human cell lines
Abstract
Background: Cathepsin H is a cysteine protease considered to play a major role in tumor progression, however, its precise function in tumorigenesis is unclear. Cathepsin H was recently proposed to be involved in processing of bone morphogenetic protein 4 (BMP-4) in mice. In order to clarify whether cathepsin H also regulates BMP-4 in humans, its impact on BMP-4 expression, processing and degradation was investigated in prostate cancer (PC-3), osteosarcoma (HOS) and pro-monocytic (U937) human cell lines.
Materials and methods: BMP-4 expression was founded to be regulated by cathepsin H using PCR array technology and confirmed by real time PCR. Immunoassays including Western blot and confocal microscopy were used to evaluate the influence of cathepsin H on BMP-4 processing.
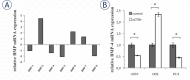
Results: In contrast to HOS, the expression of BMP-4 mRNA in U937 and PC3 cells was significantly decreased by cathepsin H. The different regulation of BMP-4 synthesis could be associated with the absence of the mature 28 kDa cathepsin H form in HOS cells, where only the intermediate 30 kDa form was observed. No co-localization of BMP-4 and cathepsin H was observed in human cell lines and the multistep processing of BMP-4 was not altered in the presence of specific cathepsin H inhibitor. Isolated cathepsin H does not cleave mature recombinant BMP-4, neither with its amino- nor its endopeptidase activity.
Conclusions: Our results exclude direct proteolytic processing of BMP-4 by cathepsin H, however, they provide support for its involvement in the regulation of BMP-4 expression.
Keywords: bone morphogenetic protein 4; cancer; cathepsin H; human cell lines; proteolytic enzymes.
Conflict of interest statement
Disclosure: No potential conflicts of interest were disclosed.
Figures


References
-
- Kirschke H, Cathepsin H. In: Handbook of proteolytic enzymes. 2nd edition. Barret AJ, Rawlings ND, Woessner JF, editors. London: Elsevier Academic Press; 2004. pp. 1089–92.
-
- Kirschke H, Barrett AJ, Rawlings ND. Lysosomal cysteine proteases. 2nd edition. Oxford: Oxford University Press; 1998.
-
- Ritonja A, Popovic T, Kotnik M, Machleidt W, Turk V. Amino acid sequences of the human kidney cathepsins H and L. FEBS Lett. 1988;228:341–5. - PubMed
-
- Vasiljeva O, Dolinar M, Turk V, Turk B. Recombinant human cathepsin H lacking the mini chain is an endopeptidase. Biochemistry. 2003;42:13522–8. - PubMed
LinkOut - more resources
Full Text Sources

