In Search for Factors that Drive Hantavirus Epidemics
- PMID: 22934002
- PMCID: PMC3429022
- DOI: 10.3389/fphys.2012.00237
In Search for Factors that Drive Hantavirus Epidemics
Abstract
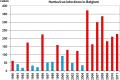
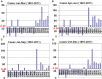
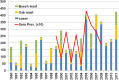
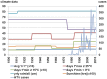
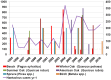
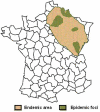
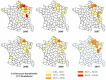
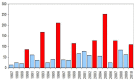
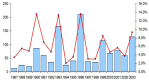
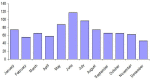
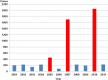
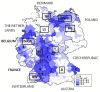
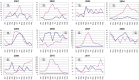
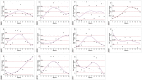
In Europe, hantaviruses (Bunyaviridae) are small mammal-associated zoonotic and emerging pathogens that can cause hemorrhagic fever with renal syndrome (HFRS). Puumala virus, the main etiological agent carried by the bank vole Myodes glareolus is responsible for a mild form of HFRS while Dobrava virus induces less frequent but more severe cases of HFRS. Since 2000 in Europe, more than 3000 cases of HFRS have been recorded, in average, each year, which is nearly double compared to the previous decade. In addition to this upside long-term trend, significant oscillations occur. Epidemic years appear, usually every 2-4 years, with an increased incidence, generally in localized hot spots. Moreover, the virus has been identified in new areas in the recent years. A great number of surveys have been carried out in order to assess the prevalence of the infection in the reservoir host and to identify links with different biotic and abiotic factors. The factors that drive the infections are related to the density and diversity of bank vole populations, prevalence of infection in the reservoir host, viral excretion in the environment, survival of the virus outside its host, and human behavior, which affect the main transmission virus route through inhalation of infected rodent excreta. At the scale of a rodent population, the prevalence of the infection increases with the age of the individuals but also other parameters, such as sex and genetic variability, interfere. The contamination of the environment may be correlated to the number of newly infected rodents, which heavily excrete the virus. The interactions between these different parameters add to the complexity of the situation and explain the absence of reliable tools to predict epidemics. In this review, the factors that drive the epidemics of hantaviruses in Middle Europe are discussed through a panorama of the epidemiological situation in Belgium, France, and Germany.
Keywords: Belgium; France; Germany; HFRS; NE; abiotic factors; biotic factors; hantavirus.
Figures
Similar articles
-
Hantaviruses and their hosts in Europe: reservoirs here and there, but not everywhere?Vector Borne Zoonotic Dis. 2010 Aug;10(6):549-61. doi: 10.1089/vbz.2009.0138. Vector Borne Zoonotic Dis. 2010. PMID: 20795916 Review.
-
Spatiotemporal dynamics of Puumala hantavirus in suburban reservoir rodent populations.J Vector Ecol. 2012 Dec;37(2):276-83. doi: 10.1111/j.1948-7134.2012.00228.x. J Vector Ecol. 2012. PMID: 23181849
-
Circulation and diagnostics of Puumala virus in Norway: nephropatia epidemica incidence and rodent population dynamics.APMIS. 2017 Aug;125(8):732-742. doi: 10.1111/apm.12712. Epub 2017 Jun 6. APMIS. 2017. PMID: 28585306
-
Model-based prediction of nephropathia epidemica outbreaks based on climatological and vegetation data and bank vole population dynamics.Zoonoses Public Health. 2013 Nov;60(7):461-77. doi: 10.1111/zph.12021. Epub 2012 Nov 26. Zoonoses Public Health. 2013. PMID: 23176630
-
The ecological dynamics of hantavirus diseases: From environmental variability to disease prevention largely based on data from China.PLoS Negl Trop Dis. 2019 Feb 21;13(2):e0006901. doi: 10.1371/journal.pntd.0006901. eCollection 2019 Feb. PLoS Negl Trop Dis. 2019. PMID: 30789905 Free PMC article. Review.
Cited by
-
Molecular and epidemiological characteristics of human Puumala and Dobrava-Belgrade hantavirus infections, Germany, 2001 to 2017.Euro Surveill. 2019 Aug;24(32):1800675. doi: 10.2807/1560-7917.ES.2019.24.32.1800675. Euro Surveill. 2019. PMID: 31411134 Free PMC article.
-
MASTREE+: Time-series of plant reproductive effort from six continents.Glob Chang Biol. 2022 May;28(9):3066-3082. doi: 10.1111/gcb.16130. Epub 2022 Mar 5. Glob Chang Biol. 2022. PMID: 35170154 Free PMC article.
-
Machine learning identifies straightforward early warning rules for human Puumala hantavirus outbreaks.Sci Rep. 2023 Mar 3;13(1):3585. doi: 10.1038/s41598-023-30596-x. Sci Rep. 2023. PMID: 36869118 Free PMC article.
-
Phylogeographic diversity of pathogenic and non-pathogenic hantaviruses in slovenia.Viruses. 2013 Dec 10;5(12):3071-87. doi: 10.3390/v5123071. Viruses. 2013. PMID: 24335778 Free PMC article.
-
Spatial dynamics of a zoonotic orthohantavirus disease through heterogenous data on rodents, rodent infections, and human disease.Sci Rep. 2019 Feb 20;9(1):2329. doi: 10.1038/s41598-019-38802-5. Sci Rep. 2019. PMID: 30787344 Free PMC article.
References
-
- Amori G., Gippoliti S. (2003). A higher–taxon approachto rodent conservation prioritiesfor the 21st century. Anim. Biodivers. Conserv. 26, 1–18
-
- RKI (2012). RKI http://www3.rki.de/Survstat/ResultList.aspx; 03/01/2012
LinkOut - more resources
Full Text Sources

