Plasticity of TRPV1-Expressing Sensory Neurons Mediating Autonomic Dysreflexia Following Spinal Cord Injury
- PMID: 22934013
- PMCID: PMC3429033
- DOI: 10.3389/fphys.2012.00257
Plasticity of TRPV1-Expressing Sensory Neurons Mediating Autonomic Dysreflexia Following Spinal Cord Injury
Abstract
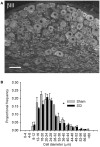
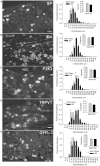
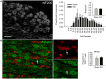
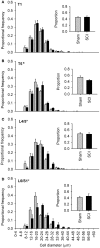
Spinal cord injury (SCI) triggers profound changes in visceral and somatic targets of sensory neurons below the level of injury. Despite this, little is known about the influence of injury to the spinal cord on sensory ganglia. One of the defining characteristics of sensory neurons is the size of their cell body: for example, nociceptors are smaller in size than mechanoreceptors or proprioceptors. In these experiments, we first used a comprehensive immunohistochemical approach to characterize the size distribution of sensory neurons after high- and low-thoracic SCI. Male Wistar rats (300 g) received a spinal cord transection (T3 or T10) or sham-injury. At 30 days post-injury, dorsal root ganglia (DRGs) and spinal cords were harvested and analyzed immunohistochemically. In a wide survey of primary afferents, only those expressing the capsaicin receptor (TRPV1) exhibited somal hypertrophy after T3 SCI. Hypertrophy only occurred caudal to SCI and was pronounced in ganglia far distal to SCI (i.e., in L4-S1 DRGs). Injury-induced hypertrophy was accompanied by a small expansion of central territory in the lumbar spinal dorsal horn and by evidence of TRPV1 upregulation. Importantly, hypertrophy of TRPV1-positive neurons was modest after T10 SCI. Given the specific effects of T3 SCI on TRPV1-positive afferents, we hypothesized that these afferents contribute to autonomic dysreflexia (AD). Rats with T3 SCI received vehicle or capsaicin via intrathecal injection at 2 or 28 days post-SCI; at 30 days, AD was assessed by recording intra-arterial blood pressure during colo-rectal distension (CRD). In both groups of capsaicin-treated animals, the severity of AD was dramatically reduced. While AD is multi-factorial in origin, TRPV1-positive afferents are clearly involved in AD elicited by CRD. These findings implicate TRPV1-positive afferents in the initiation of AD and suggest that TRPV1 may be a therapeutic target for amelioration or prevention of AD after high SCI.
Keywords: capsaicin; colo-rectal distension; dorsal horn; dorsal root; dorsal root ganglion; high blood pressure; hypertension; hypertrophy.
Figures
Similar articles
-
Paradoxical effects of continuous high dose gabapentin treatment on autonomic dysreflexia after complete spinal cord injury.Exp Neurol. 2020 Jan;323:113083. doi: 10.1016/j.expneurol.2019.113083. Epub 2019 Oct 31. Exp Neurol. 2020. PMID: 31678138 Free PMC article.
-
TRPV1 channels make major contributions to behavioral hypersensitivity and spontaneous activity in nociceptors after spinal cord injury.Pain. 2013 Oct;154(10):2130-2141. doi: 10.1016/j.pain.2013.06.040. Epub 2013 Jun 27. Pain. 2013. PMID: 23811042
-
Soluble TNFα Signaling within the Spinal Cord Contributes to the Development of Autonomic Dysreflexia and Ensuing Vascular and Immune Dysfunction after Spinal Cord Injury.J Neurosci. 2018 Apr 25;38(17):4146-4162. doi: 10.1523/JNEUROSCI.2376-17.2018. Epub 2018 Apr 2. J Neurosci. 2018. PMID: 29610439 Free PMC article.
-
How is chronic pain related to sympathetic dysfunction and autonomic dysreflexia following spinal cord injury?Auton Neurosci. 2018 Jan;209:79-89. doi: 10.1016/j.autneu.2017.01.006. Epub 2017 Jan 27. Auton Neurosci. 2018. PMID: 28161248 Free PMC article. Review.
-
Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury.Prog Brain Res. 2006;152:265-74. doi: 10.1016/S0079-6123(05)52017-X. Prog Brain Res. 2006. PMID: 16198706 Free PMC article. Review.
Cited by
-
Enhanced nociceptive behavior and expansion of associated primary afferents in a rabbit model of cerebral palsy.J Neurosci Res. 2022 Oct;100(10):1951-1966. doi: 10.1002/jnr.25108. Epub 2022 Jul 15. J Neurosci Res. 2022. PMID: 35839339 Free PMC article.
-
Development of a decerebrate model for investigating mechanisms mediating viscero-sympathetic reflexes in the spinalized rat.Am J Physiol Heart Circ Physiol. 2019 Jun 1;316(6):H1332-H1340. doi: 10.1152/ajpheart.00724.2018. Epub 2019 Mar 15. Am J Physiol Heart Circ Physiol. 2019. PMID: 30875256 Free PMC article.
-
An Autonomic Neuroprosthesis: Noninvasive Electrical Spinal Cord Stimulation Restores Autonomic Cardiovascular Function in Individuals with Spinal Cord Injury.J Neurotrauma. 2018 Feb 1;35(3):446-451. doi: 10.1089/neu.2017.5082. Epub 2017 Nov 21. J Neurotrauma. 2018. PMID: 28967294 Free PMC article. Clinical Trial.
-
Mechanisms inducing autonomic dysreflexia during urinary bladder distention in rats with spinal cord injury.Spinal Cord. 2015 Mar;53(3):190-194. doi: 10.1038/sc.2014.233. Epub 2014 Dec 23. Spinal Cord. 2015. PMID: 25535154
-
Withdrawal and restoration of central vagal afferents within the dorsal vagal complex following subdiaphragmatic vagotomy.J Comp Neurol. 2013 Oct 15;521(15):3584-99. doi: 10.1002/cne.23374. J Comp Neurol. 2013. PMID: 23749657 Free PMC article.
References
-
- Al Dera H., Habgood M. D., Furness J. B., Brock J. A. (2011). A prominent contribution of L-type Ca2+ channels to cutaneous neurovascular transmission that is revealed after spinal cord injury augments vasoconstriction. Am. J. Physiol. Heart Circ. Physiol. 302, H752–H76210.1152/ajpheart.00745.2011 - DOI - PubMed
-
- Asfaw T. S., Hypolite J., Northington G. M., Arya L. A., Wein A. J., Malykhina A. P. (2011). Acute colonic inflammation triggers detrusor instability via activation of TRPV1 receptors in a rat model of pelvic organ cross-sensitization. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1392–R140010.1152/ajpregu.00804.2010 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

