The endoplasmic reticulum stress response in aging and age-related diseases
- PMID: 22934019
- PMCID: PMC3429039
- DOI: 10.3389/fphys.2012.00263
The endoplasmic reticulum stress response in aging and age-related diseases
Abstract
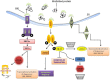
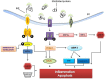
The endoplasmic reticulum(ER) is a multifunctional organelle within which protein folding, lipid biosynthesis, and calcium storage occurs. Perturbations such as energy or nutrient depletion, disturbances in calcium or redox status that disrupt ER homeostasis lead to the misfolding of proteins, ER stress and up-regulation of several signaling pathways coordinately called the unfolded protein response (UPR). The UPR is characterized by the induction of chaperones, degradation of misfolded proteins and attenuation of protein translation. The UPR plays a fundamental role in the maintenance of cellular homeostasis and thus is central to normal physiology. However, sustained unresolved ER stress leads to apoptosis. Aging linked declines in expression and activity of key ER molecular chaperones and folding enzymes compromise proper protein folding and the adaptive response of the UPR. One mechanism to explain age associated declines in cellular functions and age-related diseases is a progressive failure of chaperoning systems. In many of these diseases, proteins or fragments of proteins convert from their normally soluble forms to insoluble fibrils or plaques that accumulate in a variety of organs including the liver, brain or spleen. This group of diseases, which typically occur late in life includes Alzheimer's, Parkinson's, type II diabetes and a host of less well known but often equally serious conditions such as fatal familial insomnia. The UPR is implicated in many of these neurodegenerative and familial protein folding diseases as well as several cancers and a host of inflammatory diseases including diabetes, atherosclerosis, inflammatory bowel disease and arthritis. This review will discuss age-related changes in the ER stress response and the role of the UPR in age-related diseases.
Keywords: BiP/GRP78; UPR; age-related disease; aging; endoplasmic reticulum; stress.
Figures
References
-
- Bonnet M. H. (1985). Effect of sleep disruption on sleep, performance, and mood. Sleep 8, 11–19 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

