An integrated fluid-chemical model toward modeling the formation of intra-luminal thrombus in abdominal aortic aneurysms
- PMID: 22934022
- PMCID: PMC3429042
- DOI: 10.3389/fphys.2012.00266
An integrated fluid-chemical model toward modeling the formation of intra-luminal thrombus in abdominal aortic aneurysms
Abstract
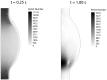
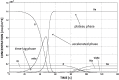
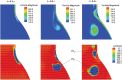
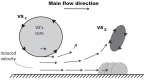
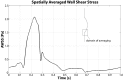
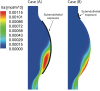
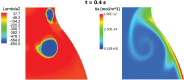
Abdominal Aortic Aneurysms (AAAs) are frequently characterized by the presence of an Intra-Luminal Thrombus (ILT) known to influence their evolution biochemically and biomechanically. The ILT progression mechanism is still unclear and little is known regarding the impact of the chemical species transported by blood flow on this mechanism. Chemical agonists and antagonists of platelets activation, aggregation, and adhesion and the proteins involved in the coagulation cascade (CC) may play an important role in ILT development. Starting from this assumption, the evolution of chemical species involved in the CC, their relation to coherent vortical structures (VSs) and their possible effect on ILT evolution have been studied. To this end a fluid-chemical model that simulates the CC through a series of convection-diffusion-reaction (CDR) equations has been developed. The model involves plasma-phase and surface-bound enzymes and zymogens, and includes both plasma-phase and membrane-phase reactions. Blood is modeled as a non-Newtonian incompressible fluid. VSs convect thrombin in the domain and lead to the high concentration observed in the distal portion of the AAA. This finding is in line with the clinical observations showing that the thickest ILT is usually seen in the distal AAA region. The proposed model, due to its ability to couple the fluid and chemical domains, provides an integrated mechanochemical picture that potentially could help unveil mechanisms of ILT formation and development.
Keywords: abdominal aortic aneurysm; coagulation cascade; computational fluid dynamics; convection-diffusion-reaction equations; intra-luminal thrombus; platelets; thrombin; vortical structures.
Figures


References
-
- Acheson D. J. (2003). Elementary Fluid Dynamics. Oxford University Press
-
- Ambrose J. A., Almeida O. D., Sharma S. K., Ratner D. G. (1997). Angiographic evolution of intra-coronary thrombus and dissection following percutaneous transluminal coronary angioplasty (the Thrombolysis and Angioplasty in Unstable Angina [TAUSA] trial). Am. J. Cardiol. 79, 559–56310.1016/S0002-9149(96)00815-6 - DOI - PubMed
-
- Antoniak S., Boltzen U., Eisenreich A., Stellbaum C., Poller W., Schultheiss H. P., Rauch U. (2009). Regulation of cardiomyocyte full-length tissue-factor expression and microparticles release under inflammatory conditions in vitro. J. Thromb. Haemost. 7, 871–87810.1111/j.1538-7836.2009.03323.x - DOI - PubMed
LinkOut - more resources
Full Text Sources

