Period2 gene mutant mice show compromised insulin-mediated endothelial nitric oxide release and altered glucose homeostasis
- PMID: 22934083
- PMCID: PMC3429102
- DOI: 10.3389/fphys.2012.00337
Period2 gene mutant mice show compromised insulin-mediated endothelial nitric oxide release and altered glucose homeostasis
Abstract
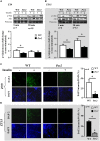
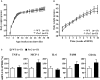
Period2 (Per2) is an important component of the circadian clock. Mutation of this gene is associated with vascular endothelial dysfunction and altered glucose metabolism. The aim of this study is to further characterize whole body glucose homeostasis and endothelial nitric oxide (NO) production in response to insulin in the mPer2(Brdm1) mice. We show that mPer2(Brdm1) mice exhibit compromised insulin receptor activation and Akt signaling in various tissues including liver, fat, heart, and aortas with a tissue-specific heterogeneous diurnal pattern, and decreased insulin-stimulated NO release in the aortas in both active and inactive phases of the animals. As compared to wild type (WT) mice, the mPer2(Brdm1) mice reveal hyperinsulinemia, hypoglycemia with lower fasting hepatic glycogen content and glycogen synthase level, no difference in glucose tolerance and insulin tolerance. The mPer2(Brdm1) mice do not show increased predisposition to obesity either on normal chow or high fat diet compared to WT controls. Thus, mice with Per2 gene mutation show altered glucose homeostasis and compromised insulin-stimulated NO release, independently of obesity.
Keywords: blood vessel; circadian gene; glucose; glycogen; insulin; nitric oxide; obesity; signaling.
Figures




References
-
- Cohen M. C., Rohtla K. M., Lavery C. E., Muller J. E., Mittleman M. A. (1997). Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am. J. Cardiol. 79, 1512–1516 - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

