Translating tolerogenic therapies to the clinic - where do we stand?
- PMID: 22934094
- PMCID: PMC3422982
- DOI: 10.3389/fimmu.2012.00254
Translating tolerogenic therapies to the clinic - where do we stand?
Abstract
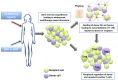
Manipulation of the immune system to prevent the development of a specific immune response is an ideal strategy to improve outcomes after transplantation. A number of experimental techniques exploiting central and peripheral tolerance mechanisms have demonstrated success, leading to the first early phase clinical trials for tolerance induction. The first major strategy centers on the facilitation of donor-cell mixed chimerism in the transplant recipient with the use of bone marrow or hematopoietic stem cell transplantation. The second strategy, utilizing peripheral regulatory mechanisms, focuses on cellular therapy with regulatory T cells. This review examines the key studies and novel research directions in the field of immunological tolerance.
Keywords: cellular therapy; chimerism; clinical trials; immune regulation; regulatory T cell; tolerance; transplantation.
Figures
References
-
- Abe M., Wang Z., De Creus A., Thomson A. W. (2005). Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival. Am. J. Transplant. 5 1808–1819 - PubMed
-
- Adeegbe D., Serafini P., Bronte V., Zoso A., Ricordi C., Inverardi L. (2010). In vivo induction of myeloid suppressor cells and CD4+Foxp3+ T regulatory cells prolongs skin allograft survival in mice. Cell Transplant. 20 941–954 - PubMed
-
- Albert M. L., Jegathesan M., Darnell R. B. (2001). Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat. Immunol. 2 1010–1017 - PubMed
-
- Ali N. A., Gaughan A. A., Orosz C. G., Baran C. P., Mcmaken S., Wang Y., Eubank T. D., Hunter M., Lichtenberger F. J., Flavahan N. A., Lawler J., Marsh C. B. (2008). Latency associated peptide has in vitro and in vivo immune effects independent of TGF-beta1. PLoS ONE 3 e1914 10.1371/journal.pone.0001914 - DOI - PMC - PubMed
-
- Allan S. E., Broady R., Gregori S., Himmel M. E., Locke N., Roncarolo M. G., Bacchetta R., Levings M. K. (2008). CD4+ T-regulatory cells: toward therapy for human diseases. Immunol. Rev. 223 391–421 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

