A prospective, multinational pharmacoepidemiological study of clinical conversion to sirolimus immunosuppression after renal transplantation
- PMID: 22934151
- PMCID: PMC3425854
- DOI: 10.1155/2012/107180
A prospective, multinational pharmacoepidemiological study of clinical conversion to sirolimus immunosuppression after renal transplantation
Abstract
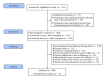
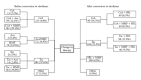
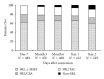
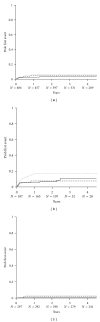
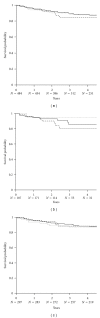
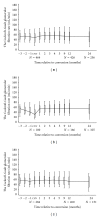
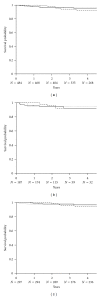
This prospective pharmacoepidemiological study examined treatment and outcomes in patients converted to sirolimus (SRL) after renal transplantation. 484 subjects in 36 centres in 7 countries were followed for up to 5 years. Principal reasons for conversion were declining graft function (146/484, 30%) and side effects of prior therapy (144/484, 30%) and the major treatment combinations after conversion were SRL ± MMF (62%), SRL + TAC (21.5%), SRL + CSA (16.5%). The cumulative probability of biopsy-confirmed acute rejection (BCAR) was 5% (n = 22), death-censored graft loss 12% (n = 56) and death 6% (n = 22), and there was no significant relationship to the treatment combination employed. Median calculated creatinine clearance was 48.4 (29.3, 64.5) mL/min at conversion, rising to 54.1 (41.2, 69.0) mL/min at month 1, 55.7 (39.0, 73.0) mL/min at month 12, 58.6 (39.7, 75.2) mL/min at two years and 60.9 (36.0, 77.0) mL/min at three years post-conversion. The most common adverse events were hypertension (47%), hyperlipidemia (26%), urinary tract infections (25%), anaemia (24%) and diarrhea (14%), and cardiac events, hyperlipemia and CMV infection were more common in patients converted during the first year. SRL was most frequently combined with MMF after conversion, but principal clinical outcomes were not significantly influenced by the treatment combination employed in normal practice.
Figures
References
-
- Law BK. Rapamycin: an anti-cancer immunosuppressant? Critical Reviews in Oncology/Hematology. 2005;56(1):47–60. - PubMed
-
- Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation. Drugs. 2007;67(3):369–391. - PubMed
-
- Neuhaus P, Klupp J, Langrehr JM. mTOR inhibitors: an overview. Liver Transplantation. 2001;7(6):473–484. - PubMed
-
- Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. Lancet. 2000;356(9225):194–202. - PubMed
-
- MacDonald AS. A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation. 2001;71(2):271–280. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

