Designing Paclitaxel drug delivery systems aimed at improved patient outcomes: current status and challenges
- PMID: 22934190
- PMCID: PMC3425796
- DOI: 10.5402/2012/623139
Designing Paclitaxel drug delivery systems aimed at improved patient outcomes: current status and challenges
Abstract
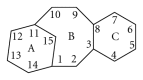
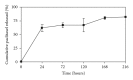
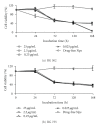
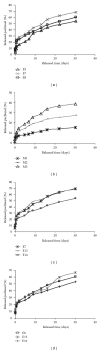
Paclitaxel is one of the most widely used and effective antineoplastic agents derived from natural sources. It has a wide spectrum of antitumor activity, particularly against ovarian cancer, breast cancer, nonsmall cell lung cancer, head and neck tumors, Kaposi's sarcoma, and urologic malignancies. It is a highly lipophilic compound with a log P value of 3.96 and very poor aqueous solubility of less than 0.01 mg/mL. In addition, the compound lacks functional groups that are ionizable which could potentially lead to an increase in its solubility with the alteration in pH. Therefore, the delivery of paclitaxel is associated with substantial challenges. Until the introduction of Abraxane, only commercial formulation was solution of paclitaxel in cremophor, which caused severe side effects. However, in recent years, a number of approaches have been reported to solubilize paclitaxel using cosolvents and inclusion complexes. In addition, innovative approaches have been reported for passive targeting of tumors using nanoparticles, nanosuspensions, liposomes, emulsions, micelles, implants, pastes and gels. All approaches for delivery of improved therapeutic outcome have been discussed in this paper.
Figures





References
-
- Farina V. The Chemistry and Pharmacology of Taxol and Its Derivatives. Elsevier Science; 1995.
-
- Kim SC, Kim DW, Shim YH, et al. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. Journal of Controlled Release. 2001;72(1–3):191–202. - PubMed
-
- Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. International Journal of Pharmaceutics. 2002;235(1-2):179–192. - PubMed
-
- McGuire WP, Rowinsky EK, Rosenhein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Annals of Internal Medicine. 1989;111(4):273–279. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
