Phage display generation of a novel human anti-CD1A monoclonal antibody with potent cytolytic activity
- PMID: 22934889
- PMCID: PMC7372535
- DOI: 10.1111/bjh.12033
Phage display generation of a novel human anti-CD1A monoclonal antibody with potent cytolytic activity
Abstract
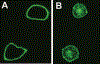
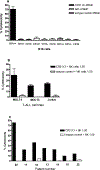
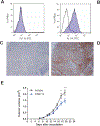
CD1A is a cell surface protein expressed on Langerhans cells and cortical thymocytes that could potentially be used as an immunotherapeutic target in Langerhans Cell Histiocytosis (LCH), the cortical subtype of T-cell acute lymphocytic leukaemia (T-ALL) and other CD1A-positive tumours. The monoclonal antibody (mAb) CR2113 was selected from a panel of six fully human mAbs isolated from a semi-synthetic phage display library, based on specificity and avidity against cells expressing CD1 antigen variants. CR2113 recognized CD1A in T-ALL cell lines and patient samples. Confocal microscopy revealed that the CR2113-CD1A complex was internalized at 37°C. Furthermore, while CR2113 induced moderate complement-dependent cytotoxicity (CDC), potent antibody-dependent cell cytotoxicity (ADCC) activity was observed against CD1A expressing cell lines as well as T-ALL cell lines and T-ALL patient samples. In vivo experiments showed that CR2113 as a naked antibody has modest but specific anti-tumour activity against CD1A-expressing tumours. CR2113 is a high-affinity human anti-CD1A mAb with significant ADCC activity. These properties make CR2113 a candidate for clinical diagnostic imaging and therapeutic targeting of LCH as well as potential use in other clinical applications.
© 2012 Blackwell Publishing Ltd.
Conflict of interest statement
Figures



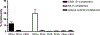
References
-
- Adams GP & Weiner LM (2005) Monoclonal antibody therapy of cancer. Nat Biotechnol, 23, 1147–1157. - PubMed
-
- Allam JP & Novak N (2006) The pathophysiology of atopic eczema. Clinical and Experimental Dermatology, 31, 89–93. - PubMed
-
- Allen CE, Li L, Peters TL, Leung HC, Yu A, Man TK, Gurusiddappa S, Phillips MT, Hicks MJ, Gaikwad A, Merad M & McClain KL (2010) Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. Journal of Immunology, 184, 4557–4567. - PMC - PubMed
-
- Amiot M, Bernard A, Raynal B, Knapp W, Deschildre C & Boumsell L (1986) Heterogeneity of the first cluster of differentiation: characterization and epitopic mapping of three CD1 molecules on normal human thymus cells. Journal of Immunology, 136, 1752–1758. - PubMed
-
- Amiot M, Dastot H, Degos L, Schmid M & Boumsell L (1987) ‘Leukocyte Typing III’. Oxford Univ Press, Oxford.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

