Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure
- PMID: 22935078
- PMCID: PMC3552442
- DOI: 10.1089/AID.2012.0089
Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure
Abstract
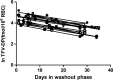
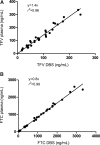
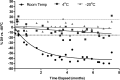
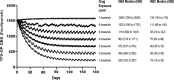
Tenofovir (TFV) disoproxil fumarate (TDF)±emtricitabine (FTC) are widely used for HIV treatment and chemoprophylaxis, but variable adherence may lead to suboptimal responses. Methods that quantify adherence would allow for interventions to improve treatment and prevention outcomes. Our objective was to characterize the pharmacokinetics of TFV-diphosphate (TFV-DP) and FTC-triphosphate (FTC-TP) in red blood cells (RBCs) and peripheral blood mononuclear cells (PBMCs); to extend the RBC analysis to dried blood spots (DBSs); and to model how RBC/DBS monitoring could inform recent and cumulative drug exposure/adherence. Blood samples were collected from 17 HIV-negative adults at 5 visits over a 30-day pharmacokinetics study of daily oral TDF/FTC. Dosing was discontinued on day 30 and blood was collected on days 35, 45, and 60 during the washout period. Plasma/RBCs/PBMCs/DBSs were all quantified by liquid chromatography/tandem mass spectrometry. DBSs were paired with RBCs and plasma for comparisons. The median (interquartile range) RBC TFV-DP half-life was 17.1 (15.7-20.2) versus 4.2 (3.7-5.2) days in PBMCs. At steady state, TFV-DP was 130 fmol/10(6) RBCs versus 98 fmol/10(6) PBMCs. FTC-TP was not quantifiable in most RBC samples. TFV-DP in RBCs versus DBSs yielded an r(2)=0.83. TFV-DP in DBSs was stable at -20°C. Simulations of TFV-DP in RBCs/DBSs, when dosed from one to seven times per week, demonstrated that each dose per week resulted in an average change of approximately 19 fmol/10(6) RBCs and 230 fmol/punch. TFV and FTC in plasma versus DBSs was defined by y=1.4x; r(2)=0.96 and y=0.8x; r(2)=0.99, respectively. We conclude that DBSs offer a convenient measure of recent (TFV/FTC) and cumulative (TFV-DP in RBCs) drug exposure with potential application to adherence monitoring.
Figures

References
-
- Lima VD. Hogg RS. Harrigan PR, et al. Continued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapy. AIDS. 2007;21:685–692. - PubMed
-
- Patel K. Hernan MA. Williams PL, et al. Pediatric AIDS Clinical Trials Group 219/219C Study Team: Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: A 10-year follow-up study. Clin Infect Dis. 2008;46:507–515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

