The apelin receptor inhibits the angiotensin II type 1 receptor via allosteric trans-inhibition
- PMID: 22935142
- PMCID: PMC3594671
- DOI: 10.1111/j.1476-5381.2012.02192.x
The apelin receptor inhibits the angiotensin II type 1 receptor via allosteric trans-inhibition
Abstract
Background and purpose: The apelin receptor (APJ) is often co-expressed with the angiotensin II type-1 receptor (AT1) and acts as an endogenous counter-regulator. Apelin antagonizes Ang II signalling, but the precise molecular mechanism has not been elucidated. Understanding this interaction may lead to new therapies for the treatment of cardiovascular disease.
Experimental approach: The physical interaction of APJ and AT1 receptors was detected by co-immunoprecipitation and bioluminescence resonance energy transfer (BRET). Functional and pharmacological interactions were measured by G-protein-dependent signalling and recruitment of β-arrestin. Allosterism and cooperativity between APJ and AT1 were measured by radioligand binding assays.
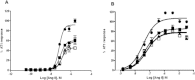
Key results: Apelin, but not Ang II, induced APJ : AT1 heterodimerization forced AT1 into a low-affinity state, reducing Ang II binding. Likewise, apelin mediated a concentration-dependent depression in the maximal production of inositol phosphate (IP(1) ) and β-arrestin recruitment to AT1 in response to Ang II. The signal depression approached a limit, the magnitude of which was governed by the cooperativity indicative of a negative allosteric interaction. Fitting the data to an operational model of allosterism revealed that apelin-mediated heterodimerization significantly reduces Ang II signalling efficacy. These effects were not observed in the absence of apelin.
Conclusions and implications: Apelin-dependent heterodimerization between APJ and AT1 causes negative allosteric regulation of AT1 function. As AT1 is significant in the pathogenesis of cardiovascular disease, these findings suggest that impaired apelin and APJ function may be a common underlying aetiology.
Linked article: This article is commented on by Goupil et al., pp. 1101-1103 of this issue. To view this commentary visit http://dx.doi.org/10.1111/bph.12040.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures






Comment in
-
GPCR heterodimers: asymmetries in ligand binding and signalling output offer new targets for drug discovery.Br J Pharmacol. 2013 Mar;168(5):1101-3. doi: 10.1111/bph.12040. Br J Pharmacol. 2013. PMID: 23121476 Free PMC article.
References
-
- AbdAlla S, Lother H, Abdel-tawab AM, Quitterer U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J Biol Chem. 2001;276:39721–39726. - PubMed
-
- Ashley E, Chun HJ, Quertermous T. Opposing cardiovascular roles for the angiotensin and apelin signaling pathways. J Mol Cell Cardiol. 2006;41:778–781. - PubMed
-
- Bai M. Dimerization of G-protein-coupled receptors: roles in signal transduction. Cell Signal. 2004;16:175–186. - PubMed
-
- Barki-Harrington L, Luttrell LM, Rockman HA. Dual inhibition of beta-adrenergic and angiotensin II receptors by a single antagonist: a functional role for receptor-receptor interaction in vivo. Circulation. 2003;108:1611–1618. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

