Effect of completion-time windows in the analysis of health-related quality of life outcomes in cancer patients
- PMID: 22935549
- PMCID: PMC3525133
- DOI: 10.1093/annonc/mds220
Effect of completion-time windows in the analysis of health-related quality of life outcomes in cancer patients
Abstract
Background: We examined if cancer patients' health-related quality of life (HRQoL) scores on the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 are affected by the specific time point, before or during treatment, at which the questionnaire is completed, and whether this could bias the overall treatment comparison analyses.
Patients and methods: A 'completion-time window' variable was created on three closed EORTC randomised control trials in lung (non-small cell lung cancer, NSCLC) and colorectal cancer (CRC) to indicate when the QLQ-30 was completed relative to chemotherapy cycle dates, defined as 'before', 'on' and 'after'. HRQoL mean scores were calculated using a linear mixed model.
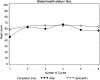
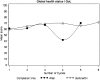
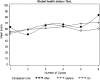
Results: Statistically significant differences (P<0.05) were observed on 6 and 5 scales for 'on' and 'after' comparisons in the NSCLC and two-group CRC trial, respectively. As for the three-group CRC trial, several statistical differences were observed in the 'before' to 'on' and the 'on' to 'after' comparisons. For all three trials, including the 'completion-time window' variable in the model resulted in a better fit, but no substantial changes in the treatment effects were noted.
Conclusions: We showed that considering the exact timing of completion within specified windows resulted in statistical and potentially clinically significant differences, but it did not alter the conclusions of treatment comparison in these studies.
Figures
References
-
- Fairclough DL. Design and Analysis of Quality of Life Studies in the Clinical Trials. London: Chapman and hall/CRC; 2002.
-
- Osoba D. Rationale for the timing of health-related quality-of-life (HQL) assessments in oncological palliative therapy. Cancer Treat Rev. 1996;22:69–73. doi:10.1016/S0305-7372(96)90066-3. - DOI - PubMed
-
- Klee MC, King MT, Machin D, et al. A clinical model for quality of life assessment in cancer patients receiving chemotherapy. Ann Oncol. 2000;11(1):23–30. doi:10.1023/A:1008394107982. - DOI - PubMed
-
- Hopwood P, Stephens RJ, Machin D. Approaches to the analysis of quality of life data: experiences gained from a medical research council lung cancer working party palliative chemotherapy trial. Qual Life Res. 1994;3(5):339–352. doi:10.1007/BF00451726. - DOI - PubMed
-
- Hürny C, Bernhard J, Coates A, et al. The International Breast Cancer Study Group: timing of baseline quality of life assessment in an international adjuvant breast cancer trial: its effect on patient self-estimation. Ann Oncol. 1994;5:65–74. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

