Macroautophagy-aided elimination of chromatin: sorting of waste, sorting of fate?
- PMID: 22935563
- PMCID: PMC3541312
- DOI: 10.4161/auto.21610
Macroautophagy-aided elimination of chromatin: sorting of waste, sorting of fate?
Abstract
How tumor cells process damaged or unwanted DNA is a matter of much interest. Recently, Rello-Varona et al. (Cell Cycle 2012; 11:170–76) reported the involvement of macroautophagy (hereon autophagy) in the elimination of micronuclei (MN) from osteosarcoma cells. Prior to that, diminution of whole nuclei from multinucleated TP53-mutant tumor cells was described. Here, we discuss these two kinds of chromatin autophagy evoked after genotoxic stress in the context of the various biological processes involved: (1) endopolyploidy and the ploidy cycle; (2) the timing of DNA synthesis; (3) DNA repair; (4) chromatin:nuclear envelope interactions; and (5) cytoplasmic autophagy. We suggest that whereas some MN can be reunited with the main nucleus (through interactions with envelope-limited chromatin sheets) and participate in DNA repair, failure of repair serves as a signal for the chromatin autophagy of MN. In turn, autophagy of whole sub-nuclei in multi-nucleated cells appears to favor de-polyploidization, mitigation of aneuploidy with its adverse effects, thereby promoting the survival fitness of descendents and treatment resistance. Thus, both kinds of chromatin autophagy provide tumor cells with the opportunity to repair DNA, sort and resort chromatin, reduce DNA content, and enhance survival.
Figures
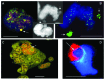
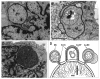
Comment on
-
Autophagic removal of micronuclei.Cell Cycle. 2012 Jan 1;11(1):170-6. doi: 10.4161/cc.11.1.18564. Epub 2012 Jan 1. Cell Cycle. 2012. PMID: 22185757
References
-
- Zybina T, Zybina E. Cell cycle modification in trophoblast cell populations in the course of placenta formation. In: Kusic-Tisma J, ed. DNA replication and related cellular processes. Rijeka, InTech, 2011:227-58.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
