Oxaliplatin/capecitabine vs oxaliplatin/infusional 5-FU in advanced colorectal cancer: the MRC COIN trial
- PMID: 22935584
- PMCID: PMC3461171
- DOI: 10.1038/bjc.2012.384
Oxaliplatin/capecitabine vs oxaliplatin/infusional 5-FU in advanced colorectal cancer: the MRC COIN trial
Abstract
Background: COIN compared first-line continuous chemotherapy with the same chemotherapy given intermittently or with cetuximab in advanced colorectal cancer (aCRC).
Methods: Choice between oxaliplatin/capecitabine (OxCap) and oxaliplatin/leucovorin (LV)/infusional 5-FU (OxFU) was by physician and patient choice and switching regimen was allowed. We compared OxCap with OxFU and OxCap+cetuximab with OxFU+cetuximab retrospectively in patients and examined efficacy, toxicity profiles and the effect of mild renal impairment.
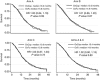
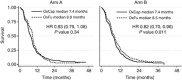
Results: In total, 64% of 2397 patients received OxCap(± cetuximab). Overall survival, progression free survival and overall response rate were similar between OxCap and OxFU but rate of radical surgeries was higher for OxFU. Progression free survival was longer for OxFU+cetuximab compared with OxCap+cetuximab but other efficacy measures were similar. Oxaliplatin/LV/infusional 5-FU (± cetuximab) was associated with more mucositis and infection whereas OxCap(± cetuximab) caused more gastrointestinal toxicities and palmar-plantar erythema. In total, 118 patients switched regimen, mainly due to toxicity; only 16% came off their second regimen due to intolerance. Patients with creatinine clearance (CrCl) 50-80 ml min(-1) on OxCap(± cetuximab) or OxFU+cetuximab had more dose modifications than those with better renal function.
Conclusions: Overall, OxFU and OxCap are equally effective in treating aCRC. However, the toxicity profiles differ and switching from one regimen to the other for poor tolerance is a reasonable option. Patients with CrCl 50-80 ml min(-1) on both regimens require close toxicity monitoring.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Adams RA, Meade AM, Seymour MT, Wilson RH, Madi A, Fisher D, Kenny SL, Kay E, Hodgkinson E, Pope M, Rogers P, Wasan H, Falk S, Gollins S, Hickish T, Bessell EM, Propper D, Kennedy MJ, Kaplan R, Maughan TS (2011) Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol 12: 642–653 - PMC - PubMed
-
- Arkenau HT, Arnold D, Cassidy J, Diaz-Rubio E, Douillard JY, Hochster H, Martoni A, Grothey A, Hinke A, Schmiegel W, Schmoll HJ, Porschen R (2008) Efficacy of oxaliplatin plus capecitabine or infusional fluorouracil/leucovorin in patients with metastatic colorectal cancer: a pooled analysis of randomized trials. J Clin Oncol 26: 5910–5917 - PubMed
-
- Braun MS, Adab F, Bradley C, McAdam K, Thomas G, Wadd NJ, Rea D, Philips R, Twelves C, Bozzino J, MacMillan C, Saunders MP, Counsell R, Anderson H, McDonald A, Stewart J, Robinson A, Davies S, Richards FJ, Seymour MT (2003) Modified de Gramont with oxaliplatin in the first-line treatment of advanced colorectal cancer. Br J Cancer 89: 1155–1158 - PMC - PubMed
-
- Cassidy J, Twelves C, Van Cutsem E, Hoff P, Bajetta E, Boyer M, Bugat R, Burger U, Garin A, Graeven U, McKendric J, Maroun J, Marshall J, Osterwalder B, Perez-Manga G, Rosso R, Rougier P, Schilsky RL (2002) First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol 13: 566–575 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous