Identification of an estrogen-regulated circadian mechanism necessary for breast acinar morphogenesis
- PMID: 22935699
- PMCID: PMC3478319
- DOI: 10.4161/cc.21946
Identification of an estrogen-regulated circadian mechanism necessary for breast acinar morphogenesis
Abstract
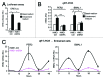
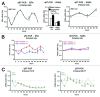
Altered estrogen receptor α (ERA) signaling and altered circadian rhythms are both features of breast cancer. By using a method to entrain circadian oscillations in human cultured cells, we recently reported that the expression of key clock genes oscillates in a circadian fashion in ERA-positive breast epithelial cells but not in breast cancer cells, regardless of their ERA status. Moreover, we reported that ERA mRNA oscillates in a circadian fashion in ERA-positive breast epithelial cells, but not in ERA-positive breast cancer cells. By using ERA-positive HME1 breast epithelial cells, which can be both entrained in vitro and can form mammary gland-like acinar structures in three-dimensional (3D) culture, first we identified a circuit encompassing ERA and an estrogen-regulated loop consisting of two circadian clock genes, PER2 and BMAL1. Further, we demonstrated that this estrogen-regulated circuit is necessary for breast epithelial acinar morphogenesis. Disruption of this circuit due to ERA-knockdown, negatively affects the estrogen-sustained circadian PER2-BMAL1 mechanism as well as the formation of 3D HME1 acini. Conversely, knockdown of either PER2 or BMAL1, by hampering the PER2-BMAL1 loop of the circadian clock, negatively affects ERA circadian oscillations and 3D breast acinar morphogenesis. To our knowledge, this study provides the first evidence of the implication of an ERA-circadian clock mechanism in the breast acinar morphogenetic process.
Figures




Similar articles
-
Circadian oscillations persist in low malignancy breast cancer cells.Cell Cycle. 2019 Oct;18(19):2447-2453. doi: 10.1080/15384101.2019.1648957. Epub 2019 Aug 11. Cell Cycle. 2019. PMID: 31357909 Free PMC article.
-
Influence of the estrous cycle on clock gene expression in reproductive tissues: effects of fluctuating ovarian steroid hormone levels.Steroids. 2010 Mar;75(3):203-12. doi: 10.1016/j.steroids.2010.01.007. Epub 2010 Jan 22. Steroids. 2010. PMID: 20096720 Free PMC article.
-
Two coupled circadian oscillations regulate Bmal1-ELuc and Per2-SLR2 expression in the mouse suprachiasmatic nucleus.Sci Rep. 2018 Oct 3;8(1):14765. doi: 10.1038/s41598-018-32516-w. Sci Rep. 2018. PMID: 30283092 Free PMC article.
-
Disruption of central and peripheral circadian clocks and circadian controlled estrogen receptor rhythms in night shift nurses in working environments.FASEB J. 2024 Jun 15;38(11):e23719. doi: 10.1096/fj.202302261RR. FASEB J. 2024. PMID: 38837828 Free PMC article.
-
[The roles of clock genes in obesity].Nihon Rinsho. 2013 Feb;71(2):244-8. Nihon Rinsho. 2013. PMID: 23631200 Review. Japanese.
Cited by
-
Circadian oscillations persist in low malignancy breast cancer cells.Cell Cycle. 2019 Oct;18(19):2447-2453. doi: 10.1080/15384101.2019.1648957. Epub 2019 Aug 11. Cell Cycle. 2019. PMID: 31357909 Free PMC article.
-
Effect of miR-34a on the expression of clock and clock-controlled genes in DLD1 and Lovo human cancer cells with different backgrounds with respect to p53 functionality and 17β-estradiol-mediated regulation.PLoS One. 2023 Oct 13;18(10):e0292880. doi: 10.1371/journal.pone.0292880. eCollection 2023. PLoS One. 2023. PMID: 37831728 Free PMC article.
-
The role of circadian rhythm in breast cancer.Chin J Cancer Res. 2013 Aug;25(4):442-50. doi: 10.3978/j.issn.1000-9604.2013.08.19. Chin J Cancer Res. 2013. PMID: 23997531 Free PMC article.
-
Altered Circadian Rhythms and Breast Cancer: From the Human to the Molecular Level.Front Endocrinol (Lausanne). 2018 May 4;9:219. doi: 10.3389/fendo.2018.00219. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29780357 Free PMC article. Review.
-
Circadian rhythms and breast cancer: from molecular level to therapeutic advancements.J Cancer Res Clin Oncol. 2024 Sep 12;150(9):419. doi: 10.1007/s00432-024-05917-w. J Cancer Res Clin Oncol. 2024. PMID: 39266868 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
