VDAC3 regulates centriole assembly by targeting Mps1 to centrosomes
- PMID: 22935710
- PMCID: PMC3478317
- DOI: 10.4161/cc.21927
VDAC3 regulates centriole assembly by targeting Mps1 to centrosomes
Abstract
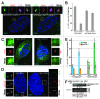
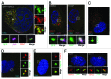
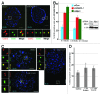
Centrioles are duplicated during S-phase to generate the two centrosomes that serve as mitotic spindle poles during mitosis. The centrosomal pool of the Mps1 kinase is important for centriole assembly, but how Mps1 is delivered to centrosomes is unknown. Here we have identified a centrosome localization domain within Mps1 and identified the mitochondrial porin VDAC3 as a protein that binds to this region of Mps1. Moreover, we show that VDAC3 is present at the mother centriole and modulates centriole assembly by recruiting Mps1 to centrosomes.
Figures


Similar articles
-
VDAC3 and Mps1 negatively regulate ciliogenesis.Cell Cycle. 2013 Mar 1;12(5):849-58. doi: 10.4161/cc.23824. Epub 2013 Feb 6. Cell Cycle. 2013. PMID: 23388454 Free PMC article.
-
Mps1 phosphorylation sites regulate the function of centrin 2 in centriole assembly.Mol Biol Cell. 2010 Dec;21(24):4361-72. doi: 10.1091/mbc.E10-04-0298. Epub 2010 Oct 27. Mol Biol Cell. 2010. PMID: 20980622 Free PMC article.
-
Antizyme restrains centrosome amplification by regulating the accumulation of Mps1 at centrosomes.Mol Biol Cell. 2010 Nov 15;21(22):3878-89. doi: 10.1091/mbc.E10-04-0281. Epub 2010 Sep 22. Mol Biol Cell. 2010. PMID: 20861309 Free PMC article.
-
Leader of the SAC: molecular mechanisms of Mps1/TTK regulation in mitosis.Open Biol. 2018 Aug;8(8):180109. doi: 10.1098/rsob.180109. Open Biol. 2018. PMID: 30111590 Free PMC article. Review.
-
The PLK4-STIL-SAS-6 module at the core of centriole duplication.Biochem Soc Trans. 2016 Oct 15;44(5):1253-1263. doi: 10.1042/BST20160116. Biochem Soc Trans. 2016. PMID: 27911707 Free PMC article. Review.
Cited by
-
Quantitative immunofluorescence assay to measure the variation in protein levels at centrosomes.J Vis Exp. 2014 Dec 20;(94):52030. doi: 10.3791/52030. J Vis Exp. 2014. PMID: 25548932 Free PMC article.
-
Identification of a new aggressive axis driven by ciliogenesis and absence of VDAC1-ΔC in clear cell Renal Cell Carcinoma patients.Theranostics. 2020 Feb 3;10(6):2696-2713. doi: 10.7150/thno.41001. eCollection 2020. Theranostics. 2020. PMID: 32194829 Free PMC article.
-
Modular elements of the TPR domain in the Mps1 N terminus differentially target Mps1 to the centrosome and kinetochore.Proc Natl Acad Sci U S A. 2016 Jul 12;113(28):7828-33. doi: 10.1073/pnas.1607421113. Epub 2016 Jun 23. Proc Natl Acad Sci U S A. 2016. PMID: 27339139 Free PMC article.
-
Centrin 3 is an inhibitor of centrosomal Mps1 and antagonizes centrin 2 function.Mol Biol Cell. 2015 Nov 1;26(21):3741-53. doi: 10.1091/mbc.E14-07-1248. Epub 2015 Sep 9. Mol Biol Cell. 2015. PMID: 26354417 Free PMC article.
-
MPS1 is involved in the HPV16-E7-mediated centrosomes amplification.Cell Div. 2021 Nov 4;16(1):6. doi: 10.1186/s13008-021-00074-9. Cell Div. 2021. PMID: 34736484 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
