Versatile approach to α-alkoxy carbamate synthesis and stimulus-responsive alcohol release
- PMID: 22936329
- PMCID: PMC3532511
- DOI: 10.1039/c2ob26571k
Versatile approach to α-alkoxy carbamate synthesis and stimulus-responsive alcohol release
Abstract
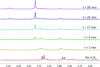
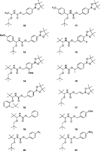
A series of α-alkoxy carbamates that cleave under mild conditions to release alcohols has been synthesized through a multicomponent process. The relationship between structural features in these compounds and the rate of alcohol release in the presence of basic hydrogen peroxide has been studied. The preparation of carbamates that cleave under other conditions has been demonstrated.
Figures
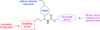










References
-
- Blencowe CA, Russell AT, Greco F, Hayes W, Thornthwaite DW. Polymer Chem. 2011;2:773.
- Shabat D, Amir RJ, Gopin A, Pessah N, Shamis M. Chem.–Eur. J. 2004;10:2626. - PubMed
-
-
For examples of alcohol and thiol release through N-acyl aminal cleavage, see: Böhm G, Dowden J, Rice DC, Burgess I, Pilard J-F, Guilbert B, Haxton A, Hunter RC, Turner NJ, Flitsch SL. Tetrahedron Lett. 1998;39:3819. Meyer Y, Richard J-A, Delest B, Noack P, Renard P-Y, Romieu A. Org. Biomol. Chem. 2010;8:1777.
-
-
- Gopin A, Pessah N, Shamis M, Rader C, Shabat D. Angew. Chem., Int. Ed. 2003;42:327. - PubMed
-
- Lee M-R, Baek K-H, Jin HJ, Jung Y-G, Shin I. Angew. Chem., Int. Ed. 2004;43:1675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

