Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation
- PMID: 22936357
- PMCID: PMC3530219
- DOI: 10.1164/rccm.201204-0754OC
Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation
Abstract
Rationale: Extracellular matrix (ECM) is a dynamic tissue that contributes to organ integrity and function, and its regulation of cell phenotype is a major aspect of cell biology. However, standard in vitro culture approaches are of unclear physiologic relevance because they do not mimic the compositional, architectural, or distensible nature of a living organ. In the lung, fibroblasts exist in ECM-rich interstitial spaces and are key effectors of lung fibrogenesis.
Objectives: To better address how ECM influences fibroblast phenotype in a disease-specific manner, we developed a culture system using acellular human normal and fibrotic lungs.
Methods: Decellularization was achieved using treatment with detergents, salts, and DNase. The resultant matrices can be sectioned as uniform slices within which cells were cultured.
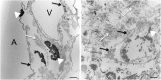
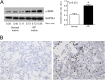
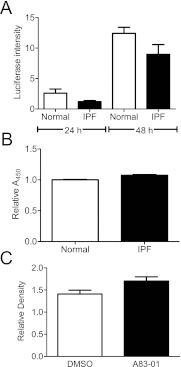
Measurements and main results: We report that the decellularization process effectively removes cellular and nuclear material while retaining native dimensionality and stiffness of lung tissue. We demonstrate that lung fibroblasts reseeded into acellular lung matrices can be subsequently assayed using conventional protocols; in this manner we show that fibrotic matrices clearly promote transforming growth factor-β-independent myofibroblast differentiation compared with normal matrices. Furthermore, comprehensive analysis of acellular matrix ECM details significant compositional differences between normal and fibrotic lungs, paving the way for further study of novel hypotheses.
Conclusions: This methodology is expected to allow investigation of important ECM-based hypotheses in human tissues and permits future scientific exploration in an organ- and disease-specific manner.
Figures







Comment in
-
The fibrotic matrix in control: does the extracellular matrix drive progression of idiopathic pulmonary fibrosis?Am J Respir Crit Care Med. 2012 Nov 1;186(9):814-6. doi: 10.1164/rccm.201208-1561ED. Am J Respir Crit Care Med. 2012. PMID: 23118079 No abstract available.
References
-
- Mutsaers SE, Bishop JE, McGrouther G, Laurent GJ. Mechanisms of tissue repair: from wound healing to fibrosis. Int J Biochem Cell Biol 1997;29:5–17 - PubMed
-
- Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature 2003;423:876–881 - PubMed
-
- Schuger L, Skubitz AP, de las Morenas A, Gilbride K. Two separate domains of laminin promote lung organogenesis by different mechanisms of action. Dev Biol 1995;169:520–532 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

