A gain-of-function polymorphism controlling complex traits and fitness in nature
- PMID: 22936775
- PMCID: PMC3872477
- DOI: 10.1126/science.1221636
A gain-of-function polymorphism controlling complex traits and fitness in nature
Abstract
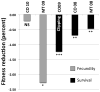
Identification of the causal genes that control complex trait variation remains challenging, limiting our appreciation of the evolutionary processes that influence polymorphisms in nature. We cloned a quantitative trait locus that controls plant defensive chemistry, damage by insect herbivores, survival, and reproduction in the natural environments where this polymorphism evolved. These ecological effects are driven by duplications in the BCMA (branched-chain methionine allocation) loci controlling this variation and by two selectively favored amino acid changes in the glucosinolate-biosynthetic cytochrome P450 proteins that they encode. These changes cause a gain of novel enzyme function, modulated by allelic differences in catalytic rate and gene copy number. Ecological interactions in diverse environments likely contribute to the widespread polymorphism of this biochemical function.
Figures


References
-
- Barrett RD, Hoekstra HE. Molecular spandrels: tests of adaptation at the genetic level. Nat Rev Genet. 2011;12:767. - PubMed
-
- Halkier BA, Gershenzon J. Biology and biochemistry of glucosinolates. Ann Rev Plant Biol. 2006;57:303. - PubMed
-
- Hopkins RJ, van Dam NM, van Loon JJA. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Ann Rev Entomol. 2009;54:57. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

