Resveratrol modulates interleukin-1β-induced phosphatidylinositol 3-kinase and nuclear factor κB signaling pathways in human tenocytes
- PMID: 22936809
- PMCID: PMC3488075
- DOI: 10.1074/jbc.M112.377028
Resveratrol modulates interleukin-1β-induced phosphatidylinositol 3-kinase and nuclear factor κB signaling pathways in human tenocytes
Abstract
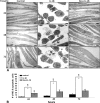
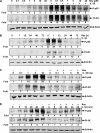
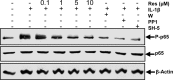
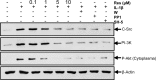
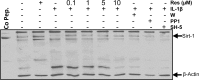
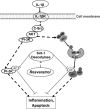
Resveratrol, an activator of histone deacetylase Sirt-1, has been proposed to have beneficial health effects due to its antioxidant and anti-inflammatory properties. However, the mechanisms underlying the anti-inflammatory effects of resveratrol and the intracellular signaling pathways involved are poorly understood. An in vitro model of human tenocytes was used to examine the mechanism of resveratrol action on IL-1β-mediated inflammatory signaling. Resveratrol suppressed IL-1β-induced activation of NF-κB and PI3K in a dose- and time-dependent manner. Treatment with resveratrol enhanced the production of matrix components collagen types I and III, tenomodulin, and tenogenic transcription factor scleraxis, whereas it inhibited gene products involved in inflammation and apoptosis. IL-1β-induced NF-κB and PI3K activation was inhibited by resveratrol or the inhibitors of PI3K (wortmannin), c-Src (PP1), and Akt (SH-5) through inhibition of IκB kinase, IκBα phosphorylation, and inhibition of nuclear translocation of NF-κB, suggesting that PI3K signaling pathway may be one of the signaling pathways inhibited by resveratrol to abrogate NF-κB activation. Inhibition of PI3K by wortmannin attenuated IL-1β-induced Akt and p65 acetylation, suggesting that p65 is a downstream component of PI3K/Akt in these responses. The modulatory effects of resveratrol on IL-1β-induced activation of NF-κB and PI3K were found to be mediated at least in part by the association between Sirt-1 and scleraxis and deacetylation of NF-κB and PI3K. Overall, these results demonstrate that activated Sirt-1 plays an essential role in the anti-inflammatory effects of resveratrol and this may be mediated at least in part through inhibition/deacetylation of PI3K and NF-κB.
Figures









References
-
- Alvares O., Klebe R., Grant G., Cochran D. L. (1995) Growth factor effects on the expression of collagenase and TIMP-1 in periodontal ligament cells. J. Periodontol. 66, 552–558 - PubMed
-
- Tewari D. S., Qian Y., Tewari M., Pieringer J., Thornton R. D., Taub R., Mochan E. O. (1994) Mechanistic features associated with induction of metalloproteinases in human gingival fibroblasts by interleukin-1. Arch. Oral. Biol. 39, 657–664 - PubMed
-
- Baldwin A. S., Jr. (1996) The NF-κB and IκB proteins. New discoveries and insights. Annu. Rev. Immunol. 14, 649–683 - PubMed
-
- Kumar A., Takada Y., Boriek A. M., Aggarwal B. B. (2004) Nuclear factor-κB. Its role in health and disease. J. Mol. Med. 82, 434–448 - PubMed
-
- Malinin N. L., Boldin M. P., Kovalenko A. V., Wallach D. (1997) MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature 385, 540–544 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

