Overexpression of vesicle-associated membrane protein (VAMP) 3, but not VAMP2, protects glucose transporter (GLUT) 4 protein translocation in an in vitro model of cardiac insulin resistance
- PMID: 22936810
- PMCID: PMC3481347
- DOI: 10.1074/jbc.M112.363630
Overexpression of vesicle-associated membrane protein (VAMP) 3, but not VAMP2, protects glucose transporter (GLUT) 4 protein translocation in an in vitro model of cardiac insulin resistance
Abstract
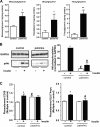
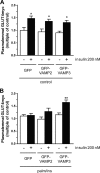
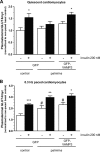
Cardiac glucose utilization is regulated by reversible translocation of the glucose transporter GLUT4 from intracellular stores to the plasma membrane. During the onset of diet-induced insulin resistance, elevated lipid levels in the circulation interfere with insulin-stimulated GLUT4 translocation, leading to impaired glucose utilization. Recently, we identified vesicle-associated membrane protein (VAMP) 2 and 3 to be required for insulin- and contraction-stimulated GLUT4 translocation, respectively, in cardiomyocytes. Here, we investigated whether overexpression of VAMP2 and/or VAMP3 could protect insulin-stimulated GLUT4 translocation under conditions of insulin resistance. HL-1 atrial cardiomyocytes transiently overexpressing either VAMP2 or VAMP3 were cultured for 16 h with elevated concentrations of palmitate and insulin. Upon subsequent acute stimulation with insulin, we measured GLUT4 translocation, plasmalemmal presence of the fatty acid transporter CD36, and myocellular lipid accumulation. Overexpression of VAMP3, but not VAMP2, completely prevented lipid-induced inhibition of insulin-stimulated GLUT4 translocation. Furthermore, the plasmalemmal presence of CD36 and intracellular lipid levels remained normal in cells overexpressing VAMP3. However, insulin signaling was not retained, indicating an effect of VAMP3 overexpression downstream of PKB/Akt. Furthermore, we revealed that endogenous VAMP3 is bound by the contraction-activated protein kinase D (PKD), and contraction and VAMP3 overexpression protect insulin-stimulated GLUT4 translocation via a common mechanism. These observations indicate that PKD activates GLUT4 translocation via a VAMP3-dependent trafficking step, which pathway might be valuable to rescue constrained glucose utilization in the insulin-resistant heart.
Figures



Similar articles
-
Requirement for distinct vesicle-associated membrane proteins in insulin- and AMP-activated protein kinase (AMPK)-induced translocation of GLUT4 and CD36 in cultured cardiomyocytes.Diabetologia. 2010 Oct;53(10):2209-19. doi: 10.1007/s00125-010-1832-7. Epub 2010 Jun 26. Diabetologia. 2010. PMID: 20582536 Free PMC article.
-
Differential regulation of cardiac glucose and fatty acid uptake by endosomal pH and actin filaments.Am J Physiol Cell Physiol. 2010 Jun;298(6):C1549-59. doi: 10.1152/ajpcell.00334.2009. Epub 2010 Apr 7. Am J Physiol Cell Physiol. 2010. PMID: 20375272
-
Phosphatidylinositol 4-kinase IIIβ mediates contraction-induced GLUT4 translocation and shows its anti-diabetic action in cardiomyocytes.Cell Mol Life Sci. 2021 Mar;78(6):2839-2856. doi: 10.1007/s00018-020-03669-7. Epub 2020 Oct 22. Cell Mol Life Sci. 2021. PMID: 33090289 Free PMC article.
-
Understanding the distinct subcellular trafficking of CD36 and GLUT4 during the development of myocardial insulin resistance.Biochim Biophys Acta Mol Basis Dis. 2020 Jul 1;1866(7):165775. doi: 10.1016/j.bbadis.2020.165775. Epub 2020 Mar 21. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 32209364 Review.
-
Protein kinase-D1 and downstream signaling mechanisms involved in GLUT4 translocation in cardiac muscle.Biochim Biophys Acta Mol Cell Res. 2024 Aug;1871(6):119748. doi: 10.1016/j.bbamcr.2024.119748. Epub 2024 May 8. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 38723678 Review.
Cited by
-
Assessing Cardiac Metabolism: A Scientific Statement From the American Heart Association.Circ Res. 2016 May 13;118(10):1659-701. doi: 10.1161/RES.0000000000000097. Epub 2016 Mar 24. Circ Res. 2016. PMID: 27012580 Free PMC article. Review.
-
Augmenting Vacuolar H+-ATPase Function Prevents Cardiomyocytes from Lipid-Overload Induced Dysfunction.Int J Mol Sci. 2020 Feb 23;21(4):1520. doi: 10.3390/ijms21041520. Int J Mol Sci. 2020. PMID: 32102213 Free PMC article.
-
Hepatocyte TMEM16A Deletion Retards NAFLD Progression by Ameliorating Hepatic Glucose Metabolic Disorder.Adv Sci (Weinh). 2020 Mar 20;7(10):1903657. doi: 10.1002/advs.201903657. eCollection 2020 May. Adv Sci (Weinh). 2020. PMID: 32440483 Free PMC article.
-
Fatty Acids Prevent Hypoxia-Inducible Factor-1α Signaling Through Decreased Succinate in Diabetes.JACC Basic Transl Sci. 2018 Aug 28;3(4):485-498. doi: 10.1016/j.jacbts.2018.04.005. eCollection 2018 Aug. JACC Basic Transl Sci. 2018. PMID: 30175272 Free PMC article.
-
Induction on Insulin Resistance In Vitro.Methods Mol Biol. 2025;2894:43-51. doi: 10.1007/978-1-0716-4342-6_5. Methods Mol Biol. 2025. PMID: 39699809
References
-
- Bachmann O. P., Dahl D. B., Brechtel K., Machann J., Haap M., Maier T., Loviscach M., Stumvoll M., Claussen C. D., Schick F., Häring H. U., Jacob S. (2001) Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes 50, 2579–2584 - PubMed
-
- Griffin M. E., Marcucci M. J., Cline G. W., Bell K., Barucci N., Lee D., Goodyear L. J., Kraegen E. W., White M. F., Shulman G. I. (1999) Free fatty acid-induced insulin resistance is associated with activation of protein kinase Cθ and alterations in the insulin signaling cascade. Diabetes 48, 1270–1274 - PubMed
-
- Schrauwen P., Schrauwen-Hinderling V., Hoeks J., Hesselink M. K. (2010) Mitochondrial dysfunction and lipotoxicity. Biochim. Biophys. Acta 1801, 266–271 - PubMed
-
- Borradaile N. M., Han X., Harp J. D., Gale S. E., Ory D. S., Schaffer J. E. (2006) Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res. 47, 2726–2737 - PubMed
-
- Szczepaniak L. S., Dobbins R. L., Metzger G. J., Sartoni-D'Ambrosia G., Arbique D., Vongpatanasin W., Unger R., Victor R. G. (2003) Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn. Reson. Med. 49, 417–423 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

