Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings
- PMID: 22936892
- PMCID: PMC3419182
- DOI: 10.1371/journal.pmed.1001290
Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings
Abstract
Background: Antiretroviral regimens with simplified dosing and better safety are needed to maximize the efficiency of antiretroviral delivery in resource-limited settings. We investigated the efficacy and safety of antiretroviral regimens with once-daily compared to twice-daily dosing in diverse areas of the world.
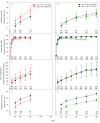
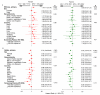
Methods and findings: 1,571 HIV-1-infected persons (47% women) from nine countries in four continents were assigned with equal probability to open-label antiretroviral therapy with efavirenz plus lamivudine-zidovudine (EFV+3TC-ZDV), atazanavir plus didanosine-EC plus emtricitabine (ATV+DDI+FTC), or efavirenz plus emtricitabine-tenofovir-disoproxil fumarate (DF) (EFV+FTC-TDF). ATV+DDI+FTC and EFV+FTC-TDF were hypothesized to be non-inferior to EFV+3TC-ZDV if the upper one-sided 95% confidence bound for the hazard ratio (HR) was ≤1.35 when 30% of participants had treatment failure. An independent monitoring board recommended stopping study follow-up prior to accumulation of 472 treatment failures. Comparing EFV+FTC-TDF to EFV+3TC-ZDV, during a median 184 wk of follow-up there were 95 treatment failures (18%) among 526 participants versus 98 failures among 519 participants (19%; HR 0.95, 95% CI 0.72-1.27; p = 0.74). Safety endpoints occurred in 243 (46%) participants assigned to EFV+FTC-TDF versus 313 (60%) assigned to EFV+3TC-ZDV (HR 0.64, CI 0.54-0.76; p<0.001) and there was a significant interaction between sex and regimen safety (HR 0.50, CI 0.39-0.64 for women; HR 0.79, CI 0.62-1.00 for men; p = 0.01). Comparing ATV+DDI+FTC to EFV+3TC-ZDV, during a median follow-up of 81 wk there were 108 failures (21%) among 526 participants assigned to ATV+DDI+FTC and 76 (15%) among 519 participants assigned to EFV+3TC-ZDV (HR 1.51, CI 1.12-2.04; p = 0.007).
Conclusion: EFV+FTC-TDF had similar high efficacy compared to EFV+3TC-ZDV in this trial population, recruited in diverse multinational settings. Superior safety, especially in HIV-1-infected women, and once-daily dosing of EFV+FTC-TDF are advantageous for use of this regimen for initial treatment of HIV-1 infection in resource-limited countries. ATV+DDI+FTC had inferior efficacy and is not recommended as an initial antiretroviral regimen.
Trial registration: www.ClinicalTrials.gov NCT00084136. Please see later in the article for the Editors' Summary.
Conflict of interest statement
TBC has received payments for lectures from, and served as a consultant for GlaxoSmithKline. TF has stock ownership in Abbot, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline and has served as a consultant for Gilead Sciences. WS is an employee of GlaxoSmithKline. JFR is an employee and stockholder of Gilead Sciences. JU is an employee of Bristol-Myers Squibb. RTS has served as a consultant to GlaxoSmithKline, Gilead Sciences, Merck and Bristol-Myers Squibb. RTS has served as a member of Data and Safety Monitoring Boards for Gilead Sciences and had research contracts with Merck and Bristol-Myers Squibb. LMS, KLK, CF, BG, MCH, JK, UL, CR, JS, MM, KS, ST, AIM, AN, AW, LM, YC, VDG and JGH declare no conflicts of interest.
Figures

Comment in
-
What is the optimal first line antiretroviral therapy in resource-limited settings?PLoS Med. 2012;9(8):e1001291. doi: 10.1371/journal.pmed.1001291. Epub 2012 Aug 14. PLoS Med. 2012. PMID: 22904690 Free PMC article.
References
-
- World Health Organization (2010) Treatment 2.0: Is this the future of treatment? World Health Organization. Available: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspubl.... Accessed 8 August 2011
-
- Hirnschall G, Schwartlander B (2011) Treatment 2.0: catalysing the next phase of scale-up. Lancet 378: 209–211. - PubMed
-
- World Health Organization (2010) Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. Geneva: World Health Organization - PubMed
-
- Staszewski S, Morales-Ramirez J, Tashima KT, Rachlis A, Skiest D, et al. (1999) Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med 341: 1865–1873. - PubMed
-
- Gulick RM, Ribaudo HJ, Shikuma CM, Lustgarten S, Squires KE, et al. (2004) Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med 350: 1850–1861. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- AI069436/AI/NIAID NIH HHS/United States
- A1069472/PHS HHS/United States
- A1069424/PHS HHS/United States
- U01 AI069513/AI/NIAID NIH HHS/United States
- AI68634/AI/NIAID NIH HHS/United States
- AI68636/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- AI050410/AI/NIAID NIH HHS/United States
- U01 AI069474/AI/NIAID NIH HHS/United States
- U01 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069513/AI/NIAID NIH HHS/United States
- AI069511/AI/NIAID NIH HHS/United States
- R01 AI047370/AI/NIAID NIH HHS/United States
- RR00865/RR/NCRR NIH HHS/United States
- AI69419/AI/NIAID NIH HHS/United States
- U01 AI069438/AI/NIAID NIH HHS/United States
- UM1 AI069426/AI/NIAID NIH HHS/United States
- AI032782/AI/NIAID NIH HHS/United States
- U01 AI027661/AI/NIAID NIH HHS/United States
- UM1 AI069476/AI/NIAID NIH HHS/United States
- RR024160/RR/NCRR NIH HHS/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- UM1 AI069421/AI/NIAID NIH HHS/United States
- AI-069439/AI/NIAID NIH HHS/United States
- U01 AI069476/AI/NIAID NIH HHS/United States
- UL1 RR024996/RR/NCRR NIH HHS/United States
- AI069476/AI/NIAID NIH HHS/United States
- P30 AI054999/AI/NIAID NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- U01 AI069417/AI/NIAID NIH HHS/United States
- AI069532/AI/NIAID NIH HHS/United States
- M01 RR000865/RR/NCRR NIH HHS/United States
- U01 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI069438/AI/NIAID NIH HHS/United States
- AI069474/AI/NIAID NIH HHS/United States
- UM1 AI069495/AI/NIAID NIH HHS/United States
- AI38858/AI/NIAID NIH HHS/United States
- AI069401/AI/NIAID NIH HHS/United States
- U01 AI069484/AI/NIAID NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- U01 AI069439/AI/NIAID NIH HHS/United States
- UM1 AI069428/AI/NIAID NIH HHS/United States
- UL1 RR024156/RR/NCRR NIH HHS/United States
- AI069513/AI/NIAID NIH HHS/United States
- TL1 RR024978/RR/NCRR NIH HHS/United States
- U01 AI069428/AI/NIAID NIH HHS/United States
- KL2 RR024977/RR/NCRR NIH HHS/United States
- U01 AI069532/AI/NIAID NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- AI27661/AI/NIAID NIH HHS/United States
- A1069467/PHS HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- UM1 AI069484/AI/NIAID NIH HHS/United States
- AI069428/AI/NIAID NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- U01 AI046376/AI/NIAID NIH HHS/United States
- UM1 AI069401/AI/NIAID NIH HHS/United States
- AI069417/AI/NIAID NIH HHS/United States
- AI069495/AI/NIAID NIH HHS/United States
- UM1 AI069518/AI/NIAID NIH HHS/United States
- AI069484/AI/NIAID NIH HHS/United States
- U01 AI069421/AI/NIAID NIH HHS/United States
- U01 AI069450/AI/NIAID NIH HHS/United States
- AI54999/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- RR024975/RR/NCRR NIH HHS/United States
- UM1 AI069399/AI/NIAID NIH HHS/United States
- AI046376/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069474/AI/NIAID NIH HHS/United States
- AI069470/AI/NIAID NIH HHS/United States
- U01 AI069518/AI/NIAID NIH HHS/United States
- AI069424/AI/NIAID NIH HHS/United States
- UL1 RR024160/RR/NCRR NIH HHS/United States
- U01 AI069495/AI/NIAID NIH HHS/United States
- U01 AI069426/AI/NIAID NIH HHS/United States
- U01 AI069399/AI/NIAID NIH HHS/United States
- AI069438/AI/NIAID NIH HHS/United States
- U01 AI069511/AI/NIAID NIH HHS/United States
- RR025747/RR/NCRR NIH HHS/United States
- AIO69463/PHS HHS/United States
- RR024156/RR/NCRR NIH HHS/United States
- AI069421/AI/NIAID NIH HHS/United States
- UM1 AI069450/AI/NIAID NIH HHS/United States
- AI69450/AI/NIAID NIH HHS/United States
- UL1 RR025747/RR/NCRR NIH HHS/United States
- A1069432/PHS HHS/United States
- UM1 AI069532/AI/NIAID NIH HHS/United States
- U01 AI069424/AI/NIAID NIH HHS/United States
- RR00424/RR/NCRR NIH HHS/United States
- U01 AI032782/AI/NIAID NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- AI045008/AI/NIAID NIH HHS/United States
- UM1 AI069417/AI/NIAID NIH HHS/United States
- AI069399/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- RR025780/RR/NCRR NIH HHS/United States
- UM1 AI069436/AI/NIAID NIH HHS/United States
- U01 AI069419/AI/NIAID NIH HHS/United States
- UM1 AI069511/AI/NIAID NIH HHS/United States
- U01 AI069401/AI/NIAID NIH HHS/United States
- AI069518/AI/NIAID NIH HHS/United States
- U01 AI069436/AI/NIAID NIH HHS/United States
- RR024996/RR/NCRR NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- AI069426/AI/NIAID NIH HHS/United States
- AI069471/AI/NIAID NIH HHS/United States
- AI47370/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

