Colesevelam hydrochloride: evidence for its use in the treatment of hypercholesterolemia and type 2 diabetes mellitus with insights into mechanism of action
- PMID: 22936894
- PMCID: PMC3426253
- DOI: 10.2147/CE.S26725
Colesevelam hydrochloride: evidence for its use in the treatment of hypercholesterolemia and type 2 diabetes mellitus with insights into mechanism of action
Abstract
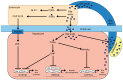
Colesevelam hydrochloride is a molecularly engineered, second-generation bile acid sequestrant demonstrating enhanced specificity for bile acids which has been approved for use as adjunctive therapy to diet and exercise as monotherapy or in combination with a β-hydroxymethylglutaryl-coenzyme A reductase inhibitor for the reduction of elevated low-density lipoprotein cholesterol in patients with primary hypercholesterolemia. It is also the only lipid-lowering agent currently available in the United States which has been approved for use as adjunctive therapy in patients with type 2 diabetes mellitus whose glycemia remains inadequately controlled on therapy with metformin, sulfonylurea, or insulin. With the recent emphasis upon drug safety by the Food and Drug Administration and various consumer agencies, it is fitting that the role of nonsystemic lipid-lowering therapies such as bile acid sequestrants - with nearly 90 years of in-class, clinically safe experience - should be reexamined. This paper presents information on the major pharmacologic effects of colesevelam, including a discussion of recent data derived from both in vitro and in vivo rodent and human studies, which shed light on the putative mechanisms involved.
Keywords: bile acid sequestrants; cholesterol; colesevelam; low-density lipoprotein.
Figures

References
-
- The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251(3):351–364. - PubMed
-
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment; prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. - PubMed
-
- Sacks FM, Moye LA, Davis BR, et al. Relationship between plasma LDL concentrations during treatment with pravastatin and recurrent coronary events in the Cholesterol and Recurrent Events trial. Circulation. 1998;97(15):1446–1452. - PubMed
-
- Davidson MH, Maki KC, Pearson TA, et al. Results of the National Cholesterol Education (NCEP) Program Evaluation Project Utilizing Novel E-Technology (NEPTUNE) II survey and implications for treatment under the recent NCEP Writing Group recommendations. Am J Cardiol. 2005;96(4):556–563. - PubMed
-
- Rosenbaum DP, Petersen JS, Ducharme S, Markham P, Goldberg DI. Absorption, distribution and excretion of GT31–104, a novel bile acid sequestrant, in rats and dogs after acute and subchronic administration. J Pharm Sci. 1997;86(5):591–595. - PubMed
LinkOut - more resources
Full Text Sources

