Using liposomes as carriers for polyphenolic compounds: the case of trans-resveratrol
- PMID: 22936976
- PMCID: PMC3425584
- DOI: 10.1371/journal.pone.0041438
Using liposomes as carriers for polyphenolic compounds: the case of trans-resveratrol
Abstract
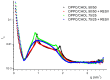
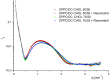
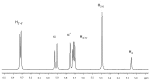
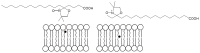
Resveratrol (3,5,4'-trihydroxy-trans-stilbene) is a polyphenol found in various plants, especially in the skin of red grapes. The effect of resveratrol on human health is the topic of numerous studies. In fact this molecule has shown anti-cancer, anti-inflammatory, blood-sugar-lowering ability and beneficial cardiovascular effects. However, for many polyphenol compounds of natural origin bioavailability is limited by low solubility in biological fluids, as well as by rapid metabolization in vivo. Therefore, appropriate carriers are required to obtain efficient therapeutics along with low administration doses.Liposomes are excellent candidates for drug delivery purposes, due to their biocompatibility, wide choice of physico-chemical properties and easy preparation.In this paper liposome formulations made by a saturated phosphatidyl-choline (DPPC) and cholesterol (or its positively charged derivative DC-CHOL) were chosen to optimize the loading of a rigid hydrophobic molecule such as resveratrol.Plain and resveratrol loaded liposomes were characterized for size, surface charge and structural details by complementary techniques, i.e. Dynamic Light Scattering (DLS), Zeta potential and Small Angle X-ray Scattering (SAXS). Nuclear and Electron Spin magnetic resonances (NMR and ESR, respectively) were also used to gain information at the molecular scale.The obtained results allowed to give an account of loaded liposomes in which resveratrol interacted with the bilayer, being more deeply inserted in cationic liposomes than in zwitterionic liposomes. Relevant properties such as the mean size and the presence of oligolamellar structures were influenced by the loading of RESV guest molecules.The toxicity of all these systems was tested on stabilized cell lines (mouse fibroblast NIH-3T3 and human astrocytes U373-MG), showing that cell viability was not affected by the administration of liposomial resveratrol.
Conflict of interest statement
Figures








References
-
- Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Natural Review of Drug Discovery 5: 493–506. - PubMed
-
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, et al. (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275: 218–220. - PubMed
-
- Aziz MH, Kumar R, Ahmad N (2003) Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (review). International Journal of Oncology 23: 17–28. - PubMed
-
- Fremont L (2000) Biological effects of resveratrol. Life Science 66: 663–673. - PubMed
-
- Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, et al. (2004) Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Research 24: 2783–2840. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

