Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers
- PMID: 22936993
- PMCID: PMC3425579
- DOI: 10.1371/journal.pone.0042821
Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers
Abstract
Introduction: In the era of malaria elimination and eradication, drug-based and vaccine-based approaches to reduce malaria transmission are receiving greater attention. Such interventions require assays that reliably measure the transmission of Plasmodium from humans to Anopheles mosquitoes.
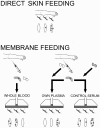
Methods: WE COMPARED TWO COMMONLY USED MOSQUITO FEEDING ASSAY PROCEDURES: direct skin feeding assays and membrane feeding assays. Three conditions under which membrane feeding assays are performed were examined: assays with i) whole blood, ii) blood pellets resuspended with autologous plasma of the gametocyte carrier, and iii) blood pellets resuspended with heterologous control serum.
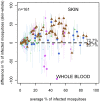
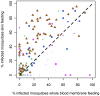
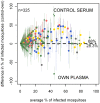
Results: 930 transmission experiments from Cameroon, The Gambia, Mali and Senegal were included in the analyses. Direct skin feeding assays resulted in higher mosquito infection rates compared to membrane feeding assays (odds ratio 2.39, 95% confidence interval 1.94-2.95) with evident heterogeneity between studies. Mosquito infection rates in membrane feeding assays and direct skin feeding assays were strongly correlated (p<0.0001). Replacing the plasma of the gametocyte donor with malaria naïve control serum resulted in higher mosquito infection rates compared to own plasma (OR 1.92, 95% CI 1.68-2.19) while the infectiousness of gametocytes may be reduced during the replacement procedure (OR 0.60, 95% CI 0.52-0.70).
Conclusions: Despite a higher efficiency of direct skin feeding assays, membrane feeding assays appear suitable tools to compare the infectiousness between individuals and to evaluate transmission-reducing interventions. Several aspects of membrane feeding procedures currently lack standardization; this variability makes comparisons between laboratories challenging and should be addressed to facilitate future testing of transmission-reducing interventions.
Conflict of interest statement
Figures



References
-
- Meis JF, Wismans PG, Jap PH, Lensen AH, Ponnudurai T (1992) A scanning electron microscopic study of the sporogonic development of Plasmodium falciparum in Anopheles stephensi. Acta Trop 50: 227–236. - PubMed
-
- Boudin C, Olivier M, Molez JF, Chiron JP, Ambroise-Thomas P (1993) High human malarial infectivity to laboratory-bred Anopheles gambiae in a village in Burkina Faso. Am J Trop Med Hyg 48: 700–706. - PubMed
-
- Schneider P, Bousema JT, Gouagna LC, Otieno S, van dV, et al. (2007) Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg 76: 470–474. - PubMed
-
- Robert V, Awono-Ambene HP, Le Hesran JY, Trape JF (2000) Gametocytemia and infectivity to mosquitoes of patients with uncomplicated Plasmodium falciparum malaria attacks treated with chloroquine or sulfadoxine plus pyrimethamine. Am J Trop Med Hyg 62: 210–216. - PubMed
-
- Graves PM (1980) Studies on the use of a membrane feeding technique for infecting Anopheles gambiae with Plasmodium falciparum. TransRSocTropMedHyg 74: 738–742. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

