Human-specific SNP in obesity genes, adrenergic receptor beta2 (ADRB2), Beta3 (ADRB3), and PPAR γ2 (PPARG), during primate evolution
- PMID: 22937051
- PMCID: PMC3427335
- DOI: 10.1371/journal.pone.0043461
Human-specific SNP in obesity genes, adrenergic receptor beta2 (ADRB2), Beta3 (ADRB3), and PPAR γ2 (PPARG), during primate evolution
Abstract
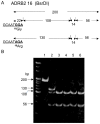
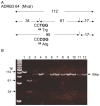
Adrenergic-receptor beta2 (ADRB2) and beta3 (ADRB3) are obesity genes that play a key role in the regulation of energy balance by increasing lipolysis and thermogenesis. The Glu27 allele in ADRB2 and the Arg64 allele in ADRB3 are associated with abdominal obesity and early onset of non-insulin-dependent diabetes mellitus (NIDDM) in many ethnic groups. Peroxisome proliferator-activated receptor γ (PPARG) is required for adipocyte differentiation. Pro12Ala mutation decreases PPARG activity and resistance to NIDDM. In humans, energy-expense alleles, Gln27 in ADRB2 and Trp64 in ADRB3, are at higher frequencies than Glu27 and Arg64, respectively, but Ala12 in PPARG is at lower frequency than Pro12. Adaptation of humans for lipolysis, thermogenesis, and reduction of fat accumulation could be considered by examining which alleles in these genes are dominant in non-human primates (NHP). All NHP (P. troglodytes, G. gorilla, P. pygmaeus, H. agilis and macaques) had energy-thrifty alleles, Gly16 and Glu27 in ADRB2, and Arg64 in ADRB3, but did not have energy-expense alleles, Arg16, Gln27 and Trp64 alleles. In PPARG gene, all NHP had large adipocyte accumulating type, the Pro12 allele.
Conclusions: These results indicate that a tendency to produce much more heat through the energy-expense alleles developed only in humans, who left tropical rainforests for savanna and developed new features in their heat-regulation systems, such as reduction of body hair and increased evaporation of water, and might have helped the protection of entrails from cold at night, especially in glacial periods.
Conflict of interest statement
Figures



Similar articles
-
Genetic polymorphisms of beta2- and beta3-adrenergic receptor genes associated with characteristics of the metabolic syndrome in black South African women.Exp Clin Endocrinol Diabetes. 2008 Apr;116(4):236-40. doi: 10.1055/s-2007-992785. Exp Clin Endocrinol Diabetes. 2008. PMID: 18393130
-
Single nucleotide variants in the beta2-adrenergic and beta3-adrenergic receptor genes explained 18.3% of adolescent obesity variation.J Hum Genet. 2005;50(7):365-369. doi: 10.1007/s10038-005-0260-x. Epub 2005 Jun 15. J Hum Genet. 2005. PMID: 15959859
-
Influence of ADRB2 Gln27Glu and ADRB3 Trp64Arg polymorphisms on body weight and body composition changes after a controlled weight-loss intervention.Appl Physiol Nutr Metab. 2016 Mar;41(3):307-14. doi: 10.1139/apnm-2015-0425. Epub 2015 Nov 25. Appl Physiol Nutr Metab. 2016. PMID: 26888112 Clinical Trial.
-
[Current trends in nutrigenomics of obesity].Vopr Pitan. 2016;85(6):6-13. Vopr Pitan. 2016. PMID: 29376303 Review. Russian.
-
beta2-Adrenergic receptor gene variations associated with stage-2 hypertension in northern Han Chinese.Ann Hum Genet. 2005 Jan;69(Pt 1):36-44. doi: 10.1046/j.1529-8817.2003.00093.x. Ann Hum Genet. 2005. PMID: 15638826 Review.
Cited by
-
Inactivation of the adrenergic receptor β2 disrupts glucose homeostasis in mice.J Endocrinol. 2014 Jun;221(3):381-90. doi: 10.1530/JOE-13-0526. J Endocrinol. 2014. PMID: 24868110 Free PMC article.
-
Functionally Significant Variants in Genes Associated with Abdominal Obesity: A Review.J Pers Med. 2023 Mar 1;13(3):460. doi: 10.3390/jpm13030460. J Pers Med. 2023. PMID: 36983642 Free PMC article. Review.
-
Systems Pharmacology-Based Method to Assess the Mechanism of Action of Weight-Loss Herbal Intervention Therapy for Obesity.Front Pharmacol. 2019 Oct 14;10:1165. doi: 10.3389/fphar.2019.01165. eCollection 2019. Front Pharmacol. 2019. PMID: 31680953 Free PMC article.
-
The crosstalk of HDAC3, microRNA-18a and ADRB3 in the progression of heart failure.Cell Biosci. 2021 Feb 6;11(1):31. doi: 10.1186/s13578-020-00523-y. Cell Biosci. 2021. PMID: 33549119 Free PMC article.
-
Obesity-related gene ADRB2, ADRB3 and GHRL polymorphisms and the response to a weight loss diet intervention in adult women.Genet Mol Biol. 2014 Mar;37(1):15-22. doi: 10.1590/s1415-47572014000100005. Epub 2013 Feb 28. Genet Mol Biol. 2014. PMID: 24688286 Free PMC article.
References
-
- Girardier L, Seydoux J (1981) Is there a sympathetic regulation of the efficiency of energy utilization? Diabetologia 20: 362–365. - PubMed
-
- Tappy L (1996) Thermic effect of food and sympathetic nervous system activity in humans. Reprod Nutr Dev 36: 391–397. - PubMed
-
- Walston J, Silver K, Bogardus C, Knowler WC, Celi FS, et al. (1995) Time of onset of non-insulin- dependent diabetes mellitus and genetic variation in the β3-adrenergic receptor gene. N Engl J Med 333: 343–347. - PubMed
-
- Widén E, Lehto M, Kanninen T, Walston J, Shuldiner AR, et al. (1995) Association of a polymorphism in the β3-adrenergic receptor gene with features of the insulin resistance syndrome in Finns. N Engl J Med 333: 348–351. - PubMed
-
- Kim-Motoyama H, Yasuda K, Yamaguchi T, Yamada N, Katakura T, et al. (1997) A mutation of the beta-3-adrenergic receptor is associated with visceral obesity but decreased serum triglyceride. Diabetologia 40: 469–472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

