Proteomic analysis reveals new cardiac-specific dystrophin-associated proteins
- PMID: 22937058
- PMCID: PMC3427372
- DOI: 10.1371/journal.pone.0043515
Proteomic analysis reveals new cardiac-specific dystrophin-associated proteins
Abstract
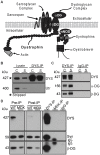
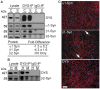
Mutations affecting the expression of dystrophin result in progressive loss of skeletal muscle function and cardiomyopathy leading to early mortality. Interestingly, clinical studies revealed no correlation in disease severity or age of onset between cardiac and skeletal muscles, suggesting that dystrophin may play overlapping yet different roles in these two striated muscles. Since dystrophin serves as a structural and signaling scaffold, functional differences likely arise from tissue-specific protein interactions. To test this, we optimized a proteomics-based approach to purify, identify and compare the interactome of dystrophin between cardiac and skeletal muscles from as little as 50 mg of starting material. We found selective tissue-specific differences in the protein associations of cardiac and skeletal muscle full length dystrophin to syntrophins and dystrobrevins that couple dystrophin to signaling pathways. Importantly, we identified novel cardiac-specific interactions of dystrophin with proteins known to regulate cardiac contraction and to be involved in cardiac disease. Our approach overcomes a major challenge in the muscular dystrophy field of rapidly and consistently identifying bona fide dystrophin-interacting proteins in tissues. In addition, our findings support the existence of cardiac-specific functions of dystrophin and may guide studies into early triggers of cardiac disease in Duchenne and Becker muscular dystrophies.
Conflict of interest statement
Figures



References
-
- Ervasti JM, Sonnemann KJ (2008) Biology of the striated muscle dystrophin-glycoprotein complex. Int Rev Cytol 265: 191–225. - PubMed
-
- Albrecht DE, Froehner SC (2002) Syntrophins and dystrobrevins: defining the dystrophin scaffold at synapses. Neurosignals 11: 123–129. - PubMed
-
- Judge DP, Kass DA, Thompson WR, Wagner KR (2011) Pathophysiology and Therapy of Cardiac Dysfunction in Duchenne Muscular Dystrophy. Am J Cardiovasc Drugs. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

