Mtb-specific CD27low CD4 T cells as markers of lung tissue destruction during pulmonary tuberculosis in humans
- PMID: 22937086
- PMCID: PMC3427145
- DOI: 10.1371/journal.pone.0043733
Mtb-specific CD27low CD4 T cells as markers of lung tissue destruction during pulmonary tuberculosis in humans
Abstract
Background: Effector CD4 T cells represent a key component of the host's anti-tuberculosis immune defense. Successful differentiation and functioning of effector lymphocytes protects the host against severe M. tuberculosis (Mtb) infection. On the other hand, effector T cell differentiation depends on disease severity/activity, as T cell responses are driven by antigenic and inflammatory stimuli released during infection. Thus, tuberculosis (TB) progression and the degree of effector CD4 T cell differentiation are interrelated, but the relationships are complex and not well understood. We have analyzed an association between the degree of Mtb-specific CD4 T cell differentiation and severity/activity of pulmonary TB infection.
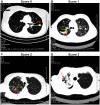
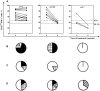
Methodology/principal findings: The degree of CD4 T cell differentiation was assessed by measuring the percentages of highly differentiated CD27(low) cells within a population of Mtb- specific CD4 T lymphocytes ("CD27(low)IFN-γ(+)" cells). The percentages of CD27(low)IFN-γ+ cells were low in healthy donors (median, 33.1%) and TB contacts (21.8%) but increased in TB patients (47.3%, p<0.0005). Within the group of patients, the percentages of CD27(low)IFN-γ(+) cells were uniformly high in the lungs (>76%), but varied in blood (12-92%). The major correlate for the accumulation of CD27(low)IFN-γ(+) cells in blood was lung destruction (r = 0.65, p = 2.7 × 10(-7)). A cutoff of 47% of CD27(low)IFN-γ(+) cells discriminated patients with high and low degree of lung destruction (sensitivity 89%, specificity 74%); a decline in CD27(low)IFN-γ(+)cells following TB therapy correlated with repair and/or reduction of lung destruction (p<0.01).
Conclusions: Highly differentiated CD27(low) Mtb-specific (CD27(low)IFN-γ(+)) CD4 T cells accumulate in the lungs and circulate in the blood of patients with active pulmonary TB. Accumulation of CD27(low)IFN-γ(+) cells in the blood is associated with lung destruction. The findings indicate that there is no deficiency in CD4 T cell differentiation during TB; evaluation of CD27(low)IFN-γ(+) cells provides a valuable means to assess TB activity, lung destruction, and tissue repair following TB therapy.
Conflict of interest statement
Figures

References
-
- Jasmer RM, Nahid P, Hopewell PC (2002) Clinical practice. Latent tuberculosis infection. N Engl J Med 347: 1860–1866. - PubMed
-
- Wallis RS, Pai M, Menzies D, Doherty TM, Walzl G, et al. (2010) Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet 375: 1920–1937. - PubMed
-
- den Hertog AL, Mayboroda OA, Klatser PR, Anthony RM (2011) Simple Rapid Near-Patient Diagnostics for Tuberculosis Remain Elusive-Is a “Treat-to-Test” Strategy More Realistic? PLoS Pathol 7. Available: http://www.plospathogens.org/article/info:doi/10.1371/journal.ppat.10022... via the Internet. Accessed 2011 Nov 3. - DOI - PMC - PubMed
-
- Ewer K, Deeks J, Alvarez L, Bryant G, Waller S, et al. (2003) Comparison of T- cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet 361: 1168–1173. - PubMed
-
- Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, et al. (2004) Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med 170: 59–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

