Mechanism-based urinary biomarkers to identify the potential for aminoglycoside-induced nephrotoxicity in premature neonates: a proof-of-concept study
- PMID: 22937100
- PMCID: PMC3427159
- DOI: 10.1371/journal.pone.0043809
Mechanism-based urinary biomarkers to identify the potential for aminoglycoside-induced nephrotoxicity in premature neonates: a proof-of-concept study
Abstract
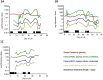
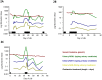
Premature infants are frequently exposed to aminoglycoside antibiotics. Novel urinary biomarkers may provide a non-invasive means for the early identification of aminoglycoside-related proximal tubule renal toxicity, to enable adjustment of treatment and identification of infants at risk of long-term renal impairment. In this proof-of-concept study, urine samples were collected from 41 premature neonates (≤ 32 weeks gestation) at least once per week, and daily during courses of gentamicin, and for 3 days afterwards. Significant increases were observed in the three urinary biomarkers measured (Kidney Injury Molecule-1 (KIM-1), Neutrophil Gelatinase-associated Lipocalin (NGAL), and N-acetyl-β-D-glucosaminidase (NAG)) during treatment with multiple courses of gentamicin. When adjusted for potential confounders, the treatment effect of gentamicin remained significant only for KIM-1 (mean difference from not treated, 1.35 ng/mg urinary creatinine; 95% CI 0.05-2.65). Our study shows that (a) it is possible to collect serial urine samples from premature neonates, and that (b) proximal tubule specific urinary biomarkers can act as indicators of aminoglycoside-associated nephrotoxicity in this age group. Further studies to investigate the clinical utility of novel urinary biomarkers in comparison to serum creatinine need to be undertaken.
Conflict of interest statement
Figures
Similar articles
-
Tubular Injury Biomarkers to Detect Gentamicin-Induced Acute Kidney Injury in the Neonatal Intensive Care Unit.Am J Perinatol. 2016 Jan;33(2):180-7. doi: 10.1055/s-0035-1563714. Epub 2015 Sep 7. Am J Perinatol. 2016. PMID: 26344007
-
Establishment of reference values for novel urinary biomarkers for renal damage in the healthy population: are age and gender an issue?Clin Chem Lab Med. 2013 Sep;51(9):1795-802. doi: 10.1515/cclm-2013-0157. Clin Chem Lab Med. 2013. PMID: 23648635
-
Urinary kidney injury molecule-1 and monocyte chemotactic protein-1 are noninvasive biomarkers of cisplatin-induced nephrotoxicity in lung cancer patients.Cancer Chemother Pharmacol. 2015 Nov;76(5):989-96. doi: 10.1007/s00280-015-2880-y. Epub 2015 Sep 25. Cancer Chemother Pharmacol. 2015. PMID: 26407820 Free PMC article. Clinical Trial.
-
Are recently reported biomarkers helpful for early and accurate diagnosis of acute kidney injury?Biomarkers. 2012 Aug;17(5):385-93. doi: 10.3109/1354750X.2012.680070. Epub 2012 Apr 19. Biomarkers. 2012. PMID: 22515481 Review.
-
Renal biomarkers of kidney injury in cardiorenal syndrome.Curr Heart Fail Rep. 2011 Jun;8(2):99-105. doi: 10.1007/s11897-011-0052-x. Curr Heart Fail Rep. 2011. PMID: 21380936 Review.
Cited by
-
Molecular nephrology: types of acute tubular injury.Nat Rev Nephrol. 2019 Oct;15(10):599-612. doi: 10.1038/s41581-019-0184-x. Epub 2019 Aug 22. Nat Rev Nephrol. 2019. PMID: 31439924 Free PMC article. Review.
-
Molecular Mechanisms and Biomarkers Associated with Chemotherapy-Induced AKI.Int J Mol Sci. 2022 Feb 27;23(5):2638. doi: 10.3390/ijms23052638. Int J Mol Sci. 2022. PMID: 35269781 Free PMC article. Review.
-
A Multiparametric Assay Platform for Simultaneous In Vivo Assessment of Pronephric Morphology, Renal Function and Heart Rate in Larval Zebrafish.Cells. 2020 May 20;9(5):1269. doi: 10.3390/cells9051269. Cells. 2020. PMID: 32443839 Free PMC article.
-
Clinical Relevance and Predictive Value of Damage Biomarkers of Drug-Induced Kidney Injury.Drug Saf. 2017 Nov;40(11):1049-1074. doi: 10.1007/s40264-017-0565-7. Drug Saf. 2017. PMID: 28674842 Review.
-
The pharmacokinetics and toxicity of morning vs. evening tobramycin dosing for pulmonary exacerbations of cystic fibrosis: A randomised comparison.J Cyst Fibros. 2016 Jul;15(4):510-7. doi: 10.1016/j.jcf.2015.07.012. Epub 2015 Aug 15. J Cyst Fibros. 2016. PMID: 26282839 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

