Probing the binding sites of antibiotic drugs doxorubicin and N-(trifluoroacetyl) doxorubicin with human and bovine serum albumins
- PMID: 22937101
- PMCID: PMC3427208
- DOI: 10.1371/journal.pone.0043814
Probing the binding sites of antibiotic drugs doxorubicin and N-(trifluoroacetyl) doxorubicin with human and bovine serum albumins
Abstract
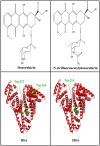
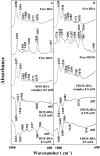
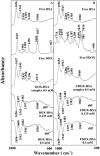
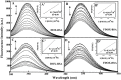
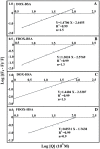
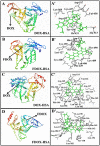
We located the binding sites of doxorubicin (DOX) and N-(trifluoroacetyl) doxorubicin (FDOX) with bovine serum albumin (BSA) and human serum albumins (HSA) at physiological conditions, using constant protein concentration and various drug contents. FTIR, CD and fluorescence spectroscopic methods as well as molecular modeling were used to analyse drug binding sites, the binding constant and the effect of drug complexation on BSA and HSA stability and conformations. Structural analysis showed that doxorubicin and N-(trifluoroacetyl) doxorubicin bind strongly to BSA and HSA via hydrophilic and hydrophobic contacts with overall binding constants of K(DOX-BSA) = 7.8 (± 0.7) × 10(3) M(-1), K(FDOX-BSA) = 4.8 (± 0.5)× 10(3) M(-1) and K(DOX-HSA) = 1.1 (± 0.3)× 10(4) M(-1), K(FDOX-HSA) = 8.3 (± 0.6)× 10(3) M(-1). The number of bound drug molecules per protein is 1.5 (DOX-BSA), 1.3 (FDOX-BSA) 1.5 (DOX-HSA), 0.9 (FDOX-HSA) in these drug-protein complexes. Docking studies showed the participation of several amino acids in drug-protein complexation, which stabilized by H-bonding systems. The order of drug-protein binding is DOX-HSA > FDOX-HSA > DOX-BSA > FDOX>BSA. Drug complexation alters protein conformation by a major reduction of α-helix from 63% (free BSA) to 47-44% (drug-complex) and 57% (free HSA) to 51-40% (drug-complex) inducing a partial protein destabilization. Doxorubicin and its derivative can be transported by BSA and HSA in vitro.
Conflict of interest statement
Figures


References
-
- Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, et al. (2009) Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem 16: 3267–3285. - PubMed
-
- Choi KC, Bang JY, Kim C, Kim PI, Lee SR, et al. (2009) Antitumor effect of adriamycin-encapsulated nanoparticles of poly(dl-lactide-co-glycolide)-grafted dextran. J Pharmacol Sci 98: 2104–2112. - PubMed
-
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56: 185–229. - PubMed
-
- Kratz F (2007) DOXO-EMCH (INNO-206): the first albumin-binding prodrug of doxorubicin to enter clinical trials. Expert Opin Invest Drugs 16: 855–866. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

