Involvement of AMPA receptor GluR2 and GluR3 trafficking in trigeminal spinal subnucleus caudalis and C1/C2 neurons in acute-facial inflammatory pain
- PMID: 22937151
- PMCID: PMC3427165
- DOI: 10.1371/journal.pone.0044055
Involvement of AMPA receptor GluR2 and GluR3 trafficking in trigeminal spinal subnucleus caudalis and C1/C2 neurons in acute-facial inflammatory pain
Abstract
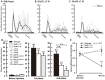
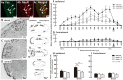
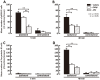
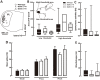
To evaluate the involvement of trafficking of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) GluR2 and GluR3 subunits in an acute inflammatory orofacial pain, we analyzed nocifensive behavior, phosphorylated extracellular signal-regulated kinase (pERK) and Fos expression in Vi/Vc, Vc and C1/C2 in GluR2 delta7 knock-in (KI), GluR3 delta7 KI mice and wild-type mice. We also studied Vc neuronal activity to address the hypothesis that trafficking of GluR2 and GluR3 subunits plays an important role in Vi/Vc, Vc and C1/C2 neuronal activity associated with orofacial inflammation in these mice. Late nocifensive behavior was significantly depressed in GluR2 delta7 KI and GluR3 delta7 KI mice. In addition, the number of pERK-immunoreactive (IR) cells was significantly decreased bilaterally in the Vi/Vc, Vc and C1/C2 in GluR2 delta7 KI and GluR3 delta7 KI mice compared to wild-type mice at 40 min after formalin injection, and was also significantly smaller in GluR3 delta7 KI compared to GluR2 delta7 KI mice. The number of Fos protein-IR cells in the ipsilateral Vi/Vc, Vc and C1/C2 was also significantly smaller in GluR2 delta7 KI and GluR3 delta7 KI mice compared to wild-type mice 40 min after formalin injection. Nociceptive neurons functionally identified as wide dynamic range neurons in the Vc, where pERK- and Fos protein-IR cell expression was prominent, showed significantly lower spontaneous activity in GluR2 delta7 KI and GluR3 delta7 KI mice than wild-type mice following formalin injection. These findings suggest that GluR2 and GluR3 trafficking is involved in the enhancement of Vi/Vc, Vc and C1/C2 nociceptive neuronal excitabilities at 16-60 min following formalin injection, resulting in orofacial inflammatory pain.
Conflict of interest statement
Figures


References
-
- Hollmann M, Heinemann S (1994) Cloned glutamate receptors. Annu Rev Neurosci 17: 31–108. - PubMed
-
- Craig AM, Blackstone CD, Huganir RL, Banker G (1993) The distribution of glutamate receptors in cultured rat hippocampal neurons: postsynaptic clustering of AMPA-selective subunits. Neuron 10: 1055–1068. - PubMed
-
- Katano T, Furue H, Okuda-Ashitaka E, Tagaya M, Watanabe M, et al. (2008) N-ethylmaleimide-sensitive fusion protein (NSF) is involved in central sensitization in the spinal cord through GluR2 subunit composition switch after inflammation. Eur J Neurosci 27: 3161–3170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

