Expression of death receptor 6 by ovarian tumors in laying hens, a preclinical model of spontaneous ovarian cancer
- PMID: 22937178
- PMCID: PMC3431036
- DOI: 10.1593/tlo.12184
Expression of death receptor 6 by ovarian tumors in laying hens, a preclinical model of spontaneous ovarian cancer
Abstract
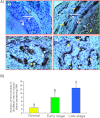
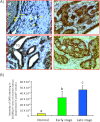
Tumor-associated neoangiogenesis and suppression of antitumor immunity are hallmarks of tumor development and progression. Death receptor 6 (DR6) has been reported to be associated with suppression of antitumor immunity and tumor progression in several malignancies. However, expression of DR6 by malignant ovarian epithelial tumors at an early stage is unknown. The goals of this study were to determine whether DR6 is expressed by malignant ovarian epithelial tumors at an early stage and to examine whether DR6 expression is associated with ovarian cancer (OVCA) progression in a laying hen model of spontaneous OVCA. Expression of DR6 was examined in normal and malignant ovaries, normal ovarian surface epithelial (OSE) cells, or malignant epithelial cells and in serum of 3-year-old hens. The population of microvessels expressing DR6 was significantly higher in hens with early-stage OVCA than hens with normal ovaries (P < .01) and increased further in late-stage OVCA. The results of this study showed that, in addition to microvessels, tumor cells in the ovary also express DR6 with a significantly higher intensity than normal OSE cells. Similar patterns of DR6 expression were also observed by immunoblot analysis and gene expression studies. Furthermore, DR6 was also detected in the serum of hens. In conclusion, DR6 expression is associated with OVCA development and progression in laying hens. This study may be helpful to examine the feasibility of DR6 as a useful surrogate marker of OVCA, a target for antitumor immunotherapy and molecular imaging and thus provide a foundation for clinical studies.
Figures


References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. - PubMed
-
- Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q, Lonning S, Teicher BA, Lee C. Tumor evasion of the immune system by converting CD4+CD25- T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-β. J Immunol. 2007;178:2883–2892. - PubMed
-
- Yigit R, Massuger LF, Figdor CG, Torensma R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol Oncol. 2010;117:366–372. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
