Pf155/RESA protein influences the dynamic microcirculatory behavior of ring-stage Plasmodium falciparum infected red blood cells
- PMID: 22937223
- PMCID: PMC3430879
- DOI: 10.1038/srep00614
Pf155/RESA protein influences the dynamic microcirculatory behavior of ring-stage Plasmodium falciparum infected red blood cells
Abstract
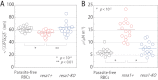
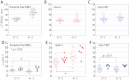
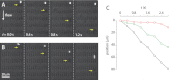
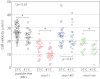
Proteins exported by Plasmodium falciparum to the red blood cell (RBC) membrane modify the structural properties of the parasitized RBC (Pf-RBC). Although quasi-static single cell assays show reduced ring-stage Pf-RBCs deformability, the parameters influencing their microcirculatory behavior remain unexplored. Here, we study the dynamic properties of ring-stage Pf-RBCs and the role of the parasite protein Pf155/Ring-Infected Erythrocyte Surface Antigen (RESA). Diffraction phase microscopy revealed RESA-driven decreased Pf-RBCs membrane fluctuations. Microfluidic experiments showed a RESA-dependent reduction in the Pf-RBCs transit velocity, which was potentiated at febrile temperature. In a microspheres filtration system, incubation at febrile temperature impaired traversal of RESA-expressing Pf-RBCs. These results show that RESA influences ring-stage Pf-RBCs microcirculation, an effect that is fever-enhanced. This is the first identification of a parasite factor influencing the dynamic circulation of young asexual Pf-RBCs in physiologically relevant conditions, offering novel possibilities for interventions to reduce parasite survival and pathogenesis in its human host.
Conflict of interest statement
M. Diez-Silva, S. Huang, J. Han, M. Dao, S. Suresh, along with others, have filed two US provisional patents on the microfluidic system. O. Puijalon, G. Deplaine, S. Perrot, along with others, have filed a patent on the microspheres filtration system.
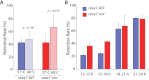
Figures

References
-
- Cooke B. M. et al. Malaria and the red blood cell membrane. Semin Hematol 41, 173–188. (2004). - PubMed
-
- Mills J. P. et al. Nonlinear elastic and viscoelastic deformation of the human red blood cell with optical tweezers. Mech Chem Biosyst 1, 169–180 (2004). - PubMed
-
- Cranston HA B. C. et al. Plasmodium falciparum maturation abolishes physiologic red cell deformability. Science 223, 400–403 (1984). - PubMed
-
- Suresh S. et al. Connections between single-cell biomechanics and human disease states: gastrointestinal cancer and malaria. Acta Biomater 1, 15–30 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

